Precision-Guided Dosing

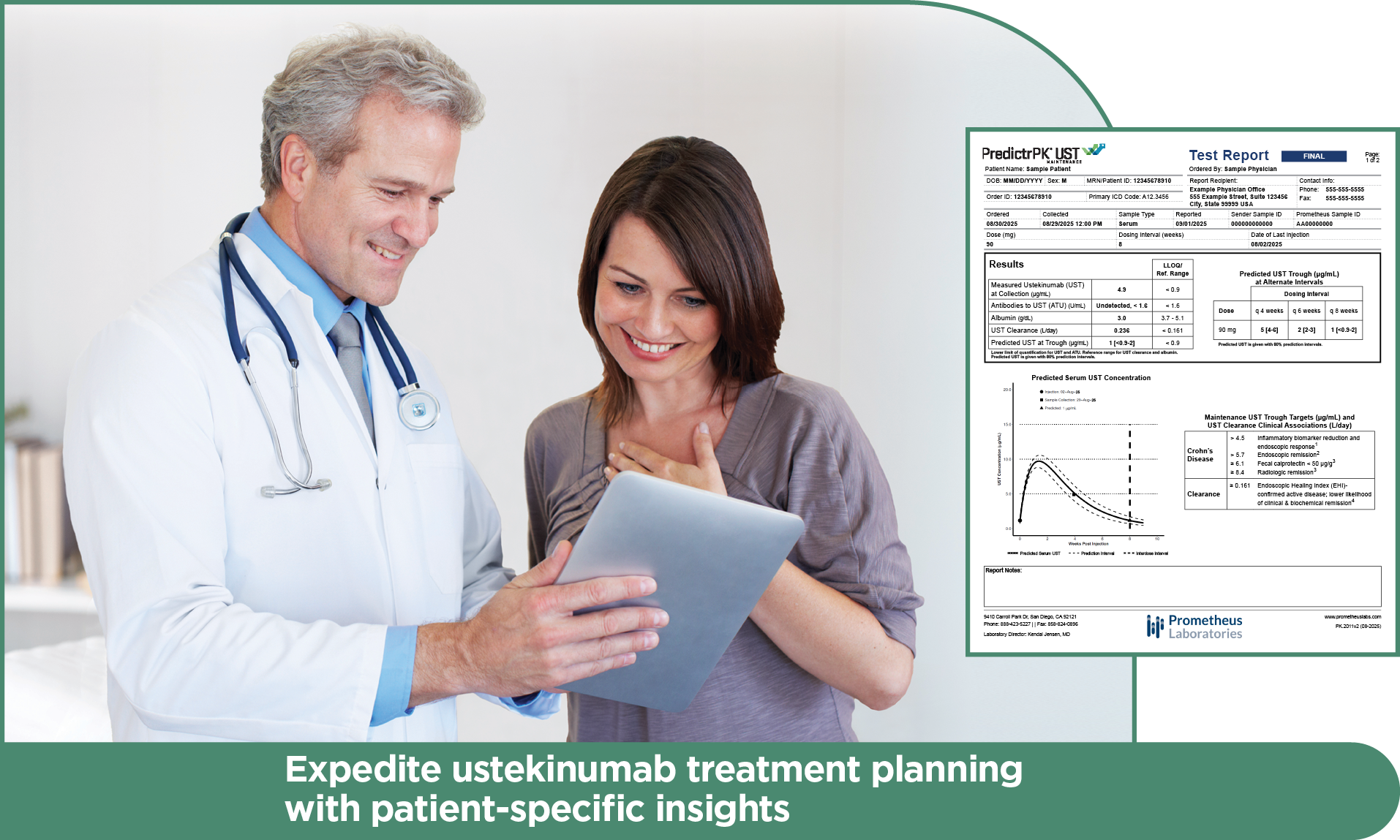
PredictrPK UST informs ustekinumab treatment decisions with precision
PredictrPK® UST leverages current dosing, serology and a proprietary population-pharmacokinetic Bayesian algorithm to deliver individualized, actionable insights supporting ustekinumab (UST) dose optimization or timely therapy changes.
Sample collection: ≤3 days prior to week 16 or, after ≥16 weeks of UST therapy, anytime ≥20 days after any maintenance injection, up to and including at trough.
Accelerated UST clearance can help identify active disease1
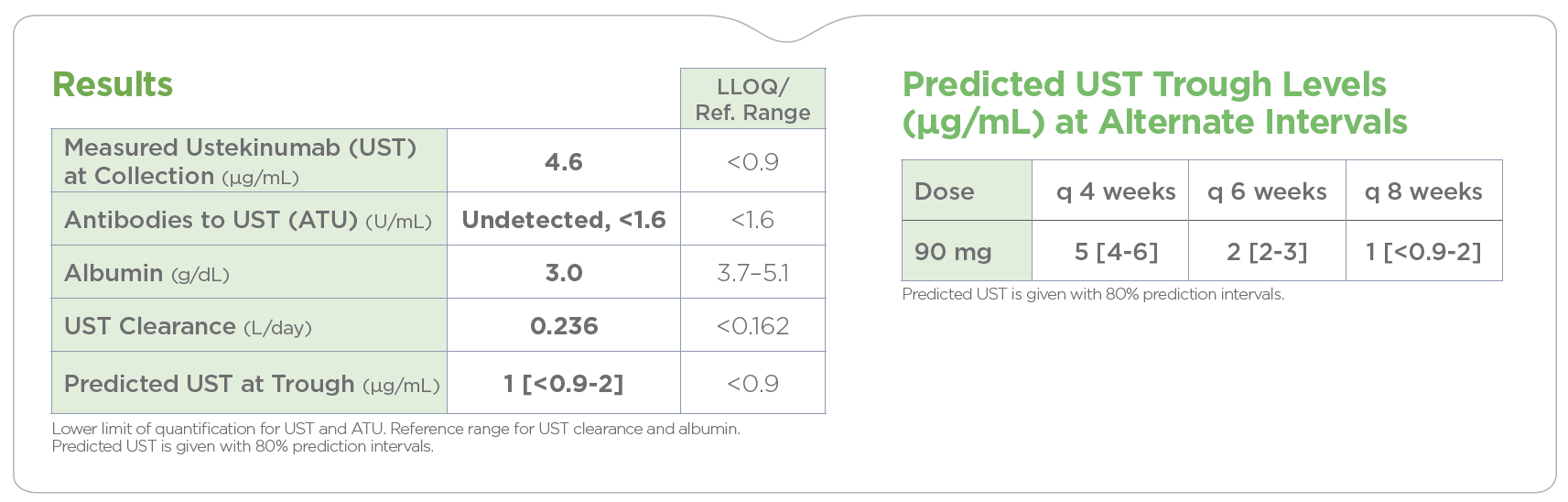
High UST clearance and low UST concentrations each associated with endoscopic healing index (EHI) scores >50, indicating a high likelihood of endoscopically active disease. Accelerated UST clearance showed better distinction in identifying active disease than UST trough concentrations alone.1
These findings indicate the clinical value of assessing UST clearance, in addition to concentrations, to improve non-invasive detection of endoscopically active disease and optimize inflammatory bowel disease (IBD) management.
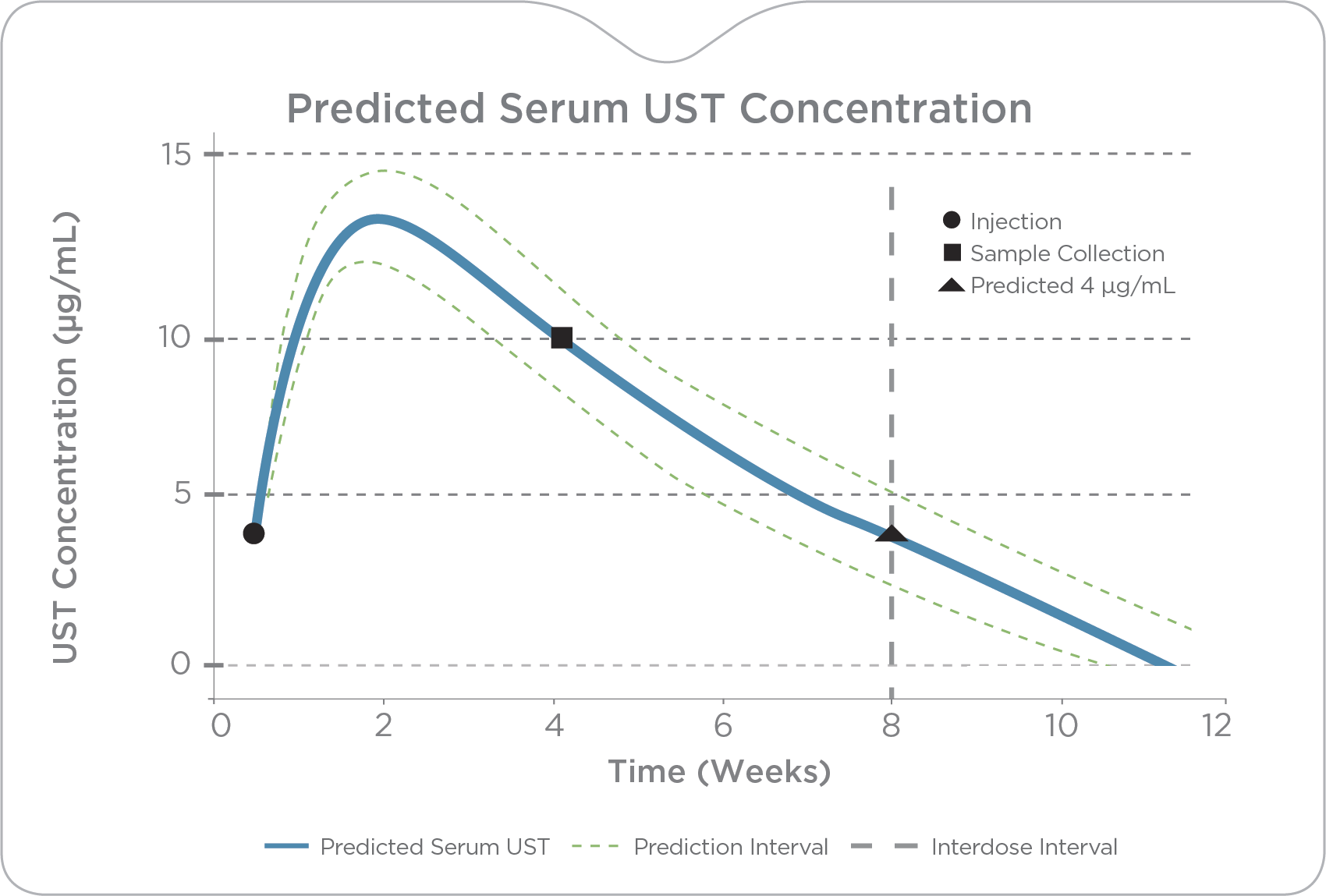
PredictrPK UST Maintenance brings a precision approach to ustekinumab therapy, enabling providers of 90 mg injection (SC) frequency based on predicted trough levels and patient-specific clearance.
By analyzing the factors known to influence drug response and durability, PredictrPK UST offers a non-invasive method to evaluate the likelihood of endoscopically active disease and guide dosing optimization via predicted trough levels at current and alternative intervals.
PredictrPK UST was developed and validated using 300+ UST cycles from 83 adult IBD patients.2
Precision-guided dosing for UST increases the likelihood of achieving therapeutic targets, recapturing clinical response and shortening the time to remission.1-2
PredictrPK is currently available for the following biologics and their respective biosimilars:
PredictrPK is part of the Prometheus continuum of care for IBD patients
PredictrPK tests are laboratory-developed tests that were developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and are performed exclusively in our high complexity CLIA-certified (05D0917432) and College of American Pathologists-accredited (6805501) clinical laboratory. As laboratory a developed tests, they have not been cleared or approved by the US FDA. These tests may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. PredictrPK UST Maintenance is validated for IBD patients ≥18 years. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Yarur AJ et al. Pharmaceutics. 2025;17(2):187.
- Data on file.








