Inflammatory Bowel Disease

Early identification improves long-term patient outcomes
Knowing patient risk factors early can change the course of IBD by identifying those likely to need advanced therapy. Evidence shows that patients treated early with biologics are five times more likely to achieve sustained steroid-free and surgery-free remission (79% vs 15%) compared to a step-up approach.18
Associations of serologic markers and disease behavior
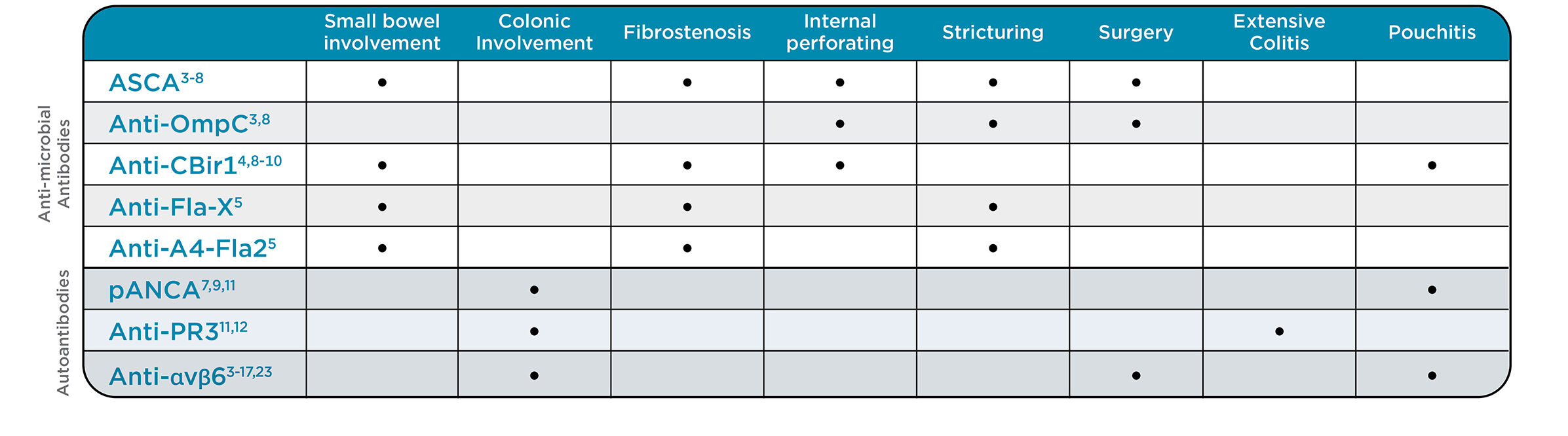
Anti-integrin (αvβ6): A novel, high-specificity marker for UC diagnosis and prognosis
- Anti-integrin (αvβ6) is a novel marker for UC that offers strong diagnostic and prognostic value, with 79% sensitivity and 94% specificity with demonstrated superiority over hs-CRP and fecal calprotectin.13
- Anti-αvβ6 supports early diagnosis as antibodies have been detected up to 10 years pre-diagnosis (12.2%), increasing to 52.4% at time of diagnosis.14
- Patients with higher concentrations of anti-αvβ6 tend to have more severe UC phenotypes.13,14,16
- This novel marker also helps identify CD patients with colonic involvement (L2 and L3).14
Anti-PR3 antibodies: Identify patients with early-onset and extensive UC
- Anti-PR3 antibodies are a specific anti-neutrophil cytoplasmic antibody (ANCA) that have been associated with early and extensive colitis, showing similar sensitivity to atypical pANCA but with higher specificity (98.1% vs. 90.4%).12
- Anti-PR3 antibodies are detected 3x more often in patients within the first 2 years of diagnosis compared to those with disease duration over 17 years, with markedly higher prevalence in E3 extensive colitis (37%) versus E2 (21%) and E1 (15%).22
Microbial antibodies: Stratify risk for complicated CD
- 65% of CD patients have developed at least one anti-microbial antibody six years before diagnosis.19
- The presence of multiple anti-microbial antibodies and higher concentrations of these antibodies correlate with the risk of more complicated disease and faster disease progression.2,3,5-8,10
- Anti-CBir1 antibodies are commonly detected in pediatric patients and can identify patients without ASCA antibodies.20
- Patients with detectable anti-FlaX antibodies have a higher risk of early post-op CD recurrence.21
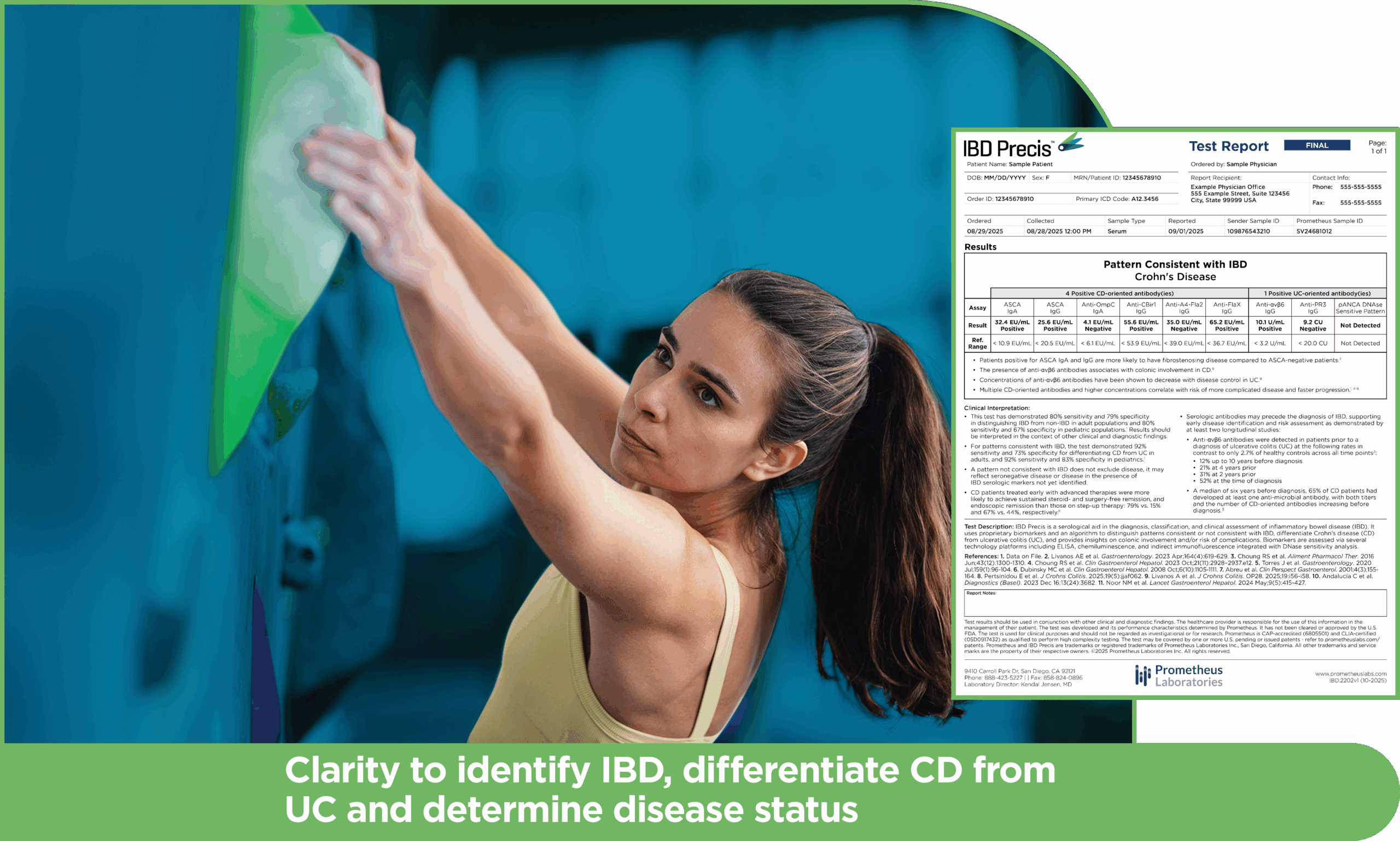
An easy-to-understand test report outlines which of these markers may apply to your patient, along with comments on clinical implications.
IBD Precis is part of the Prometheus continuum of testing solutions for IBD patients
IBD Precis is a laboratory-developed test that was developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA certified (05D0917432) and College of American Pathologists (CAP) accredited (6805501) clinical laboratory. As a laboratory developed test, it has not been cleared or approved by the US FDA. The test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. This material is provided for general information purposes only, as an educational service for healthcare providers. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Data on File.
- Choung RS et al. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2928–2937.e12.
- Mow WS et al. Gastroenterol. 2004;126(2):414-424.
- Targan SR et al. Gastroenterol. 2005 Jun;128(7):2020-2028.
- Schoepfer AM et al. Inflamm Bowel Dis. 2009 Sep;15(9):1358-1367.
- Torres J et al. Gastroenterol. 2020 Jul;159(1):96-104.
- Abreu et al. Clin Perspect Gastroenterol. 2001;4(3):155-164.
- Dubinsky MC et al. Clin Gastroenterol Hepatol. 2008 Oct;6(10):1105-1111.
- Fleshner P et al. Clin Gastroenterol Hepatol. 2008 May;6(5):561-568.
- Coukos JA et al. Dig Dis Sci. 2012 Jun;57(6):1544-1553.
- Kim JM et al. J Pediatr (Rio J). 2024 Mar-Apr;100(2):204-211.
- Andalucía C et al. Diagnostics (Basel). 2023 Dec;13(24):3682.
- Pertsinidou E et al. J Crohns Colitis. 2025 May;19(5):jjaf062.
- Livanos AE et al. Gastroenterol. 2023 Apr;164(4):619-629.
- Livanos A et al. J Crohns Colitis. OP28. 2025;19:i56-i58.
- Okabe M et al. J Gastroenterol. 2025 Jan;60(1):86-95.
- Nakanishi R et al. Inflamm Bowel Dis. 2025 Mar 3;31(3):777-785.
- Noor NM et al. Lancet Gastroenterol Hepatol. 2024 May;9(5):415-427.
- Choung RS et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1300-1310.
- Spencer EA et al. Inflamm Bowel Dis. 2018 May;24(6):1335-1343.
- Hamilton AL et al. J Gastroenterol Hepatol. 2017 Jun;32(6):1195-1203.
- Mahler M et al. Clin Chim Acta. 2013 Sep;424:267-73.
- Yanagida T et al. Intest Res. 2025 Apr. Epub ahead of print.









