Inflammatory Bowel Disease

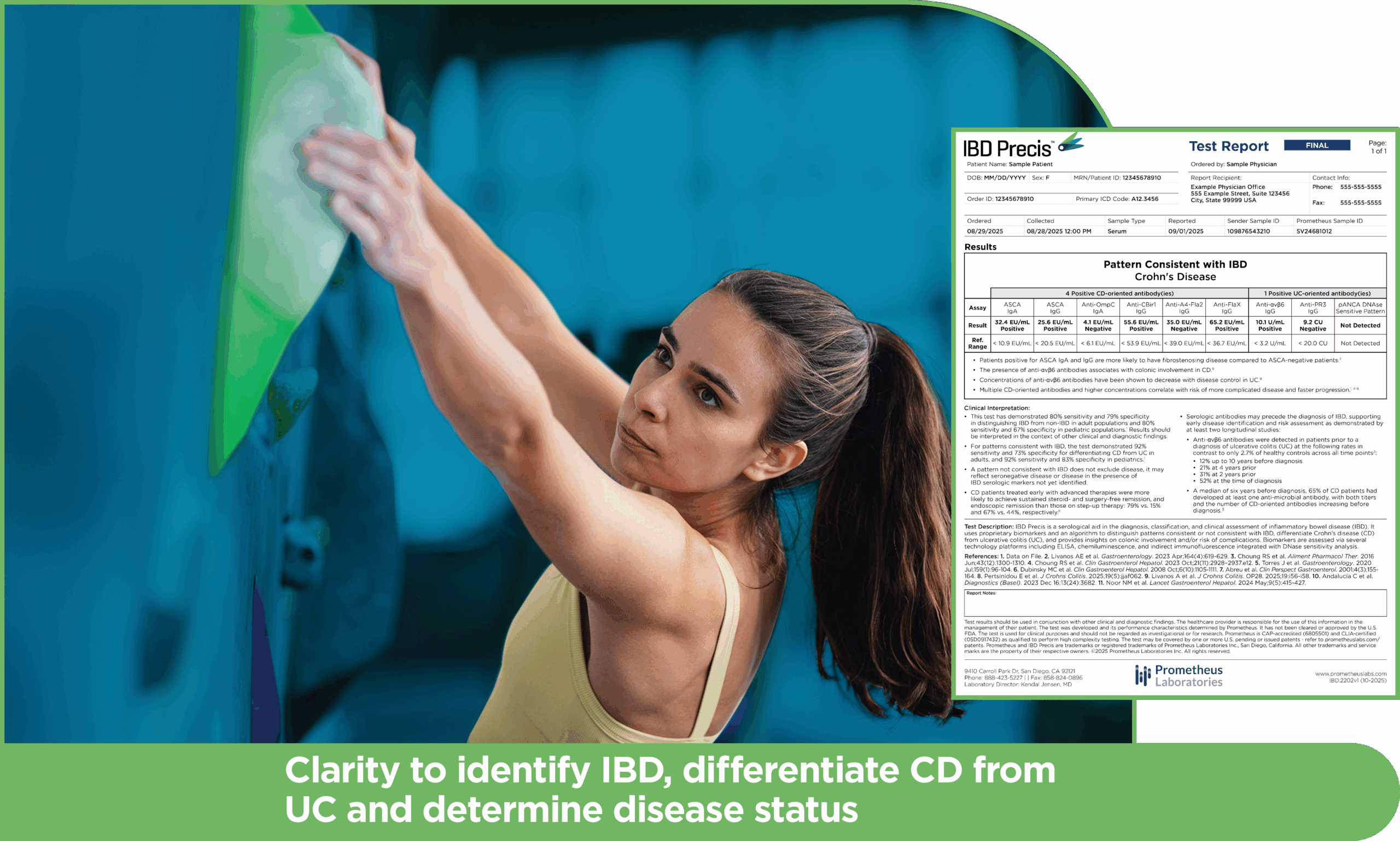
Innovation that brings clarity and individualized care to IBD assessment
On average, patients with inflammatory bowel disease experience a 16-month diagnostic delay from symptom onset. Delays in accurate inflammatory bowel disease (IBD) diagnosis can lead to disease progression, late intervention and an increased risk of emergency hospital admissions.2
Key challenges:
- Diagnostic uncertainty: Symptoms mimic other GI or infectious etiologies, leading to delayed or missed diagnosis.
- Limited non-invasive options: Current tools often rely on invasive procedures or subjective symptom reporting.
- Unclear pathology: Pathology can contradict imaging findings, adding complexity to clinical decision-making.
IBD Precis addresses these diagnostic challenges
IBD Precis is comprised of a comprehensive nine antibody panel targeting both microbial and autoantigen markers, analyzed through a proprietary algorithm that identifies serology patterns consistent and not consistent with IBD.
Key capabilities:
- Exceptional IBD rule-out: Distinguishes IBD from non-IBD with 80% sensitivity and 79% specificity in adult populations; and 80% sensitivity and 67% specificity in pediatric populations.1
- Disease differentiation: For patterns consistent with IBD, IBD Precis is 92% sensitive and 73% specific for differentiating CD from UC in adults, and 92% sensitive and 83% specific in pediatrics.1
- Location insights: Identifies colonic and/or ileal involvement.1,3,13,15,16
- Risk stratification: Predicts complication risks through unique antibody signatures.3-6,8-18
An evolution of serology enables earlier intervention and personalized care
By identifying IBD patterns missed by traditional serology, IBD Precis supports:
- Reduced diagnostic uncertainty
- Earlier disease identification
- More targeted diagnostic workups
- Personalized management strategies
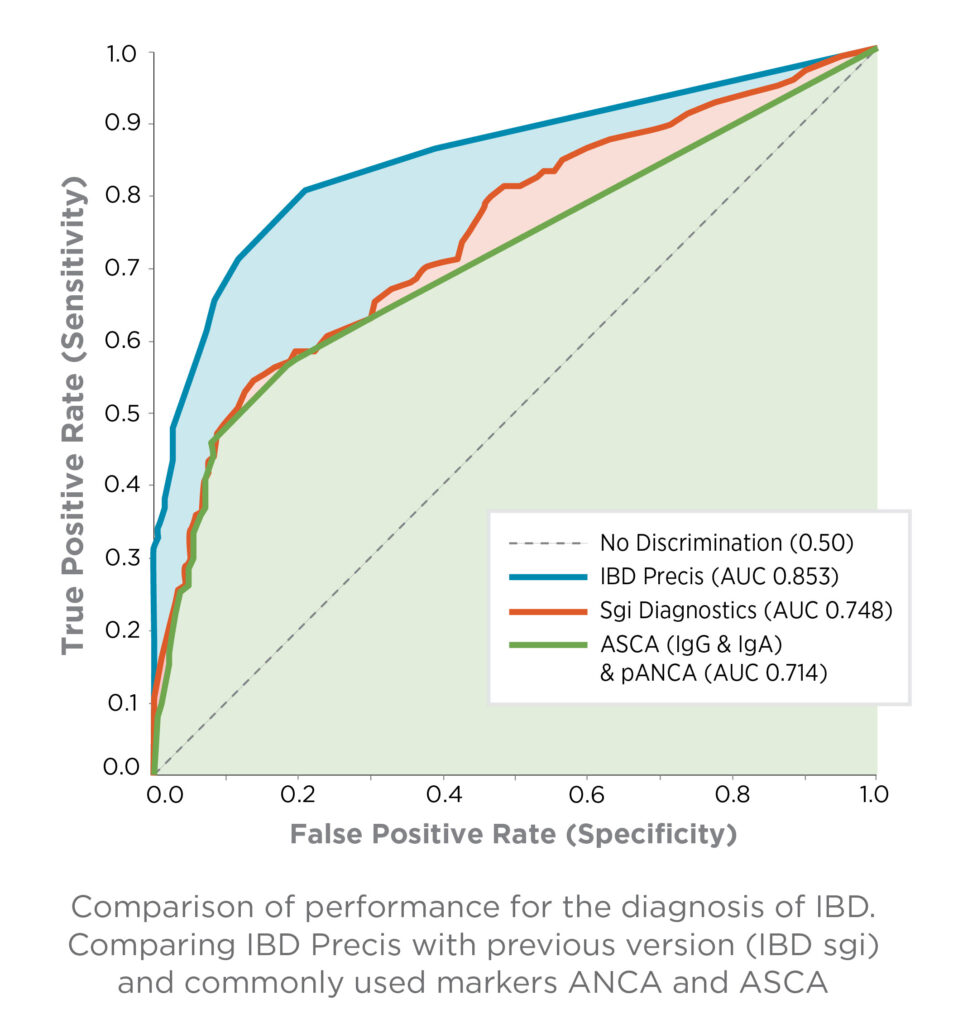
IBD Precis outperforms commonly used ANCA/ASCA markers and previous generation tests, providing the diagnostic confidence to optimize each patient’s care.1
Complementary diagnostic insights enhance IBD evaluation beyond endoscopy
IBD Precis complements endoscopic evaluation at critical decision points:
- Pre-endoscopy: Guide specialist referral decisions.
- Post-endoscopy: Clarify ambiguous or negative findings.
- Surgical planning: Inform intervention strategies.
Clinical advantages that benefit patients:
- Provides objective data for timely specialist referral.
- Detects or confirms ileal involvement to improve risk stratification of patients.
IBD Precis delivers valuable diagnostic insights that enhance clinical decision-making through the patient journey
IBD Precis helps reduce diagnostic uncertainty, accelerating clinical decision-making to support earlier initiation of targeted therapy and may help patients avoid unnecessary procedures. This enhanced diagnostic accuracy may also help reduce overall healthcare utilization.
- Enhanced diagnostic precision
- Personalized risk stratification and early disease insights
- Faster path to appropriate treatment
- Potential for reduced cost and burden for patient
IBD Precis is part of the Prometheus continuum of care for IBD patients
IBD Precis is a laboratory-developed test that was developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA certified (05D0917432) and College of American Pathologists (CAP) accredited (6805501) clinical laboratory. As a laboratory developed test, it has not been cleared or approved by the US FDA. The test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. This material is provided for general information purposes only, as an educational service for healthcare providers. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Data on File.
- Umar N et al. Inflamm Bowel Dis. 2025 Jan; 31(1):140–150.
- Choung RS et al. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2928–2937.e12.
- Mow WS et al. Gastroenterol. 2004;126(2):414-424.
- Targan SR et al. Gastroenterol. 2005 Jun;128(7):2020-2028.
- Schoepfer AM et al. Inflamm Bowel Dis. 2009 Sep;15(9):1358-1367.
- Torres J et al. Gastroenterol. 2020 Jul;159(1):96-104.
- Abreu et al. Clin Perspect Gastroenterol. 2001;4(3):155-164.
- Dubinsky MC et al. Clin Gastroenterol Hepatol. 2008 Oct;6(10):1105-1111.
- Fleshner P et al. Clin Gastroenterol Hepatol. 2008 May;6(5):561-568.
- Coukos JA et al. Dig Dis Sci. 2012 Jun;57(6):1544-1553.
- Kim JM et al. J Pediatr (Rio J). 2024 Mar-Apr;100(2):204-211.
- Andalucía C et al. Diagnostics (Basel). 2023 Dec;13(24):3682.
- Pertsinidou E et al. J Crohns Colitis. 2025 May;19(5):jjaf062.
- Livanos AE et al. Gastroenterol. 2023 Apr;164(4):619-629.
- Livanos A et al. J Crohns Colitis. OP28. 2025;19:i56-i58.
- Okabe M et al. J Gastroenterol. 2025 Jan;60(1):86-95.
- Nakanishi R et al. Inflamm Bowel Dis. 2025 Mar 3;31(3):777-785.
- Noor NM et al. Lancet Gastroenterol Hepatol. 2024 May;9(5):415-427.
- Choung RS et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1300-1310.
- Spencer EA et al. Inflamm Bowel Dis. 2018 May;24(6):1335-1343.
- Hamilton AL et al. J Gastroenterol Hepatol. 2017 Jun;32(6):1195-1203.
- Mahler M et al. Clin Chim Acta. 2013 Sep;424:267-73.
- Yanagida T et al. Intest Res. 2025 Apr. Epub ahead of print.









