Inflammatory Bowel Disease

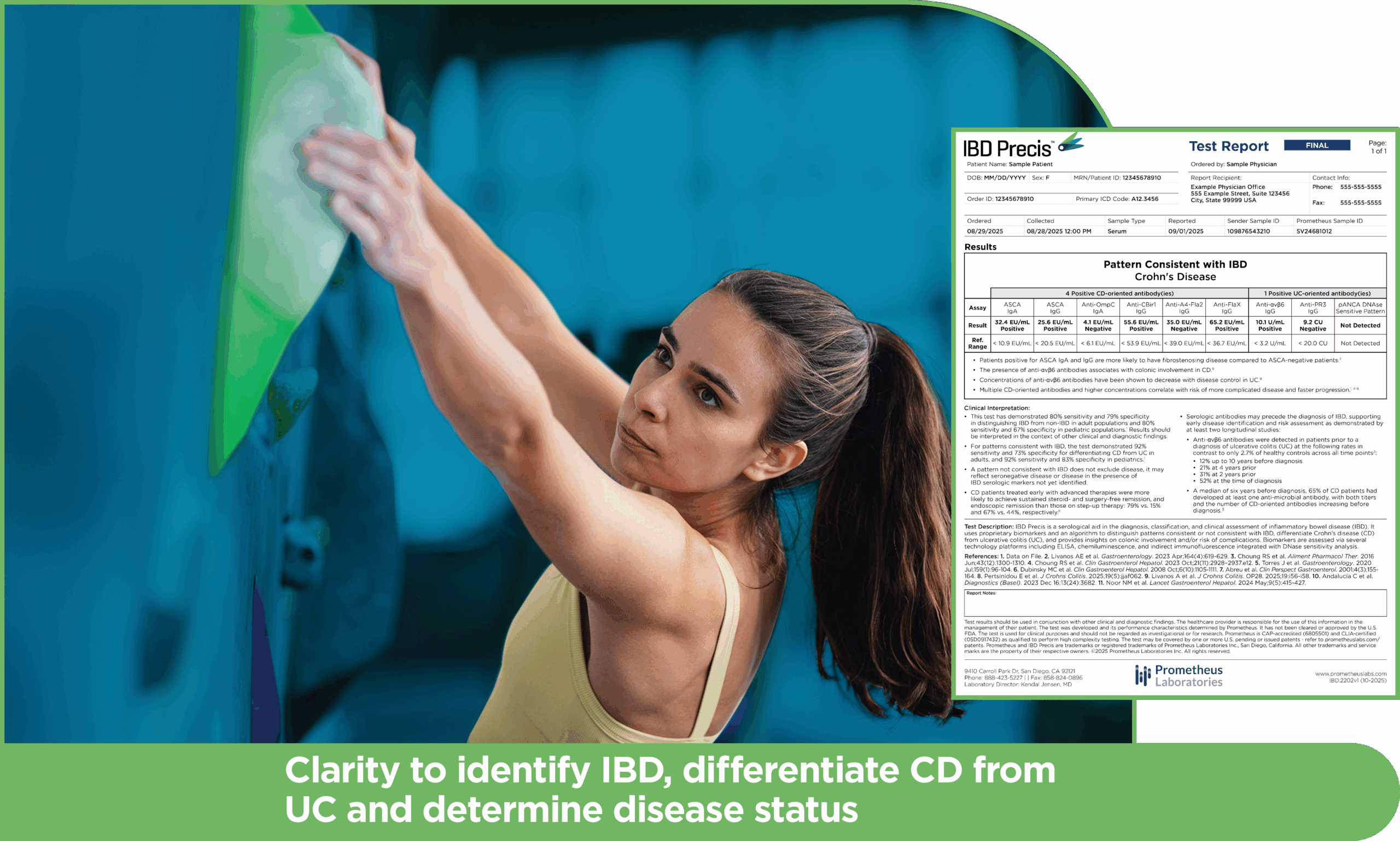
Enhanced serology results complement endoscopy to triage patients earlier and more accurately
1. Top Level Result
IBD Precis distinguishes patterns consistent or not consistent with IBD and differentiates Crohn’s disease (CD) from ulcerative colitis (UC).
*A pattern not consistent with IBD does not exclude disease, and may reflect seronegative disease or disease in the presence of IBD serologic markers not yet identified. All results should be interpreted in conjunction with other clinical and diagnostic findings.
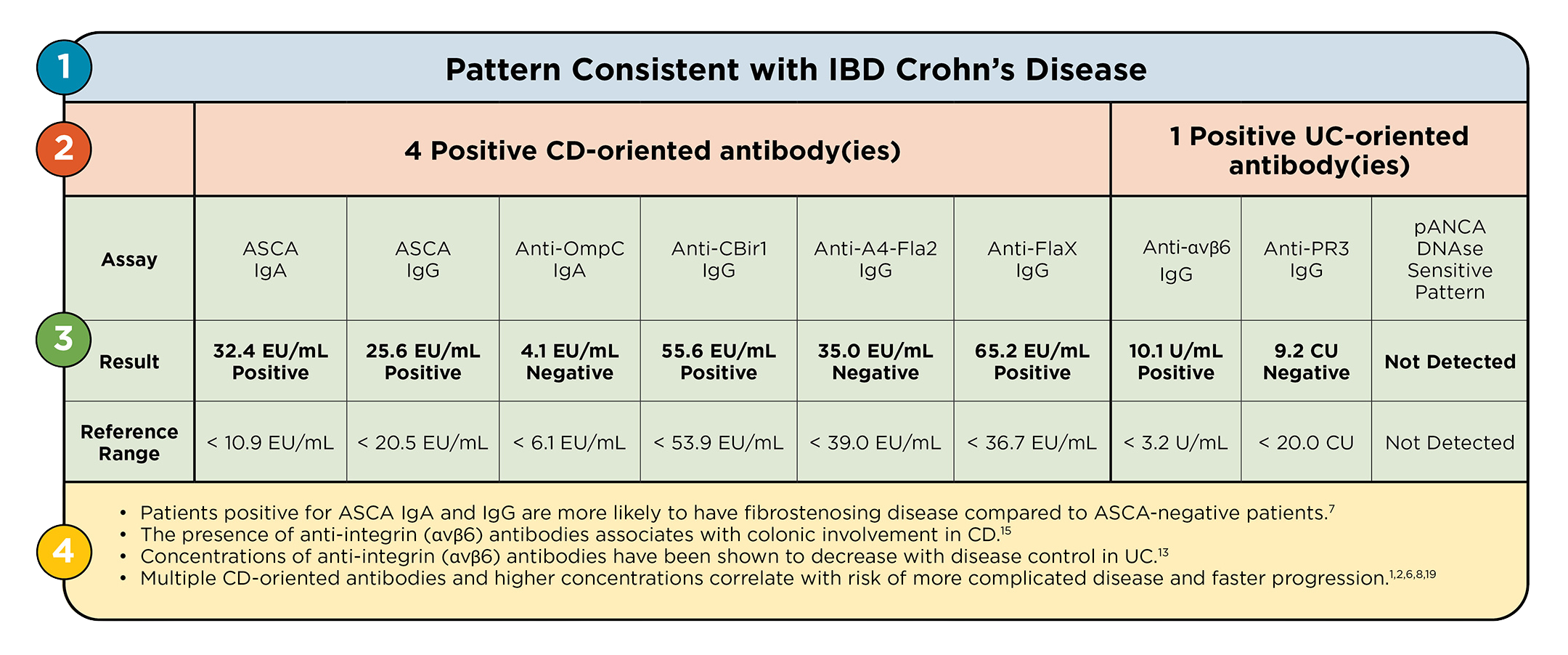
2. Individual Antibody Results
The IBD Precis assay consists of a robust panel of 9 antibodies: six CD-oriented anti-microbial antibodies associated with ileal involvement and three UC-oriented antibodies that may be autoantigens associated with colonic involvement.

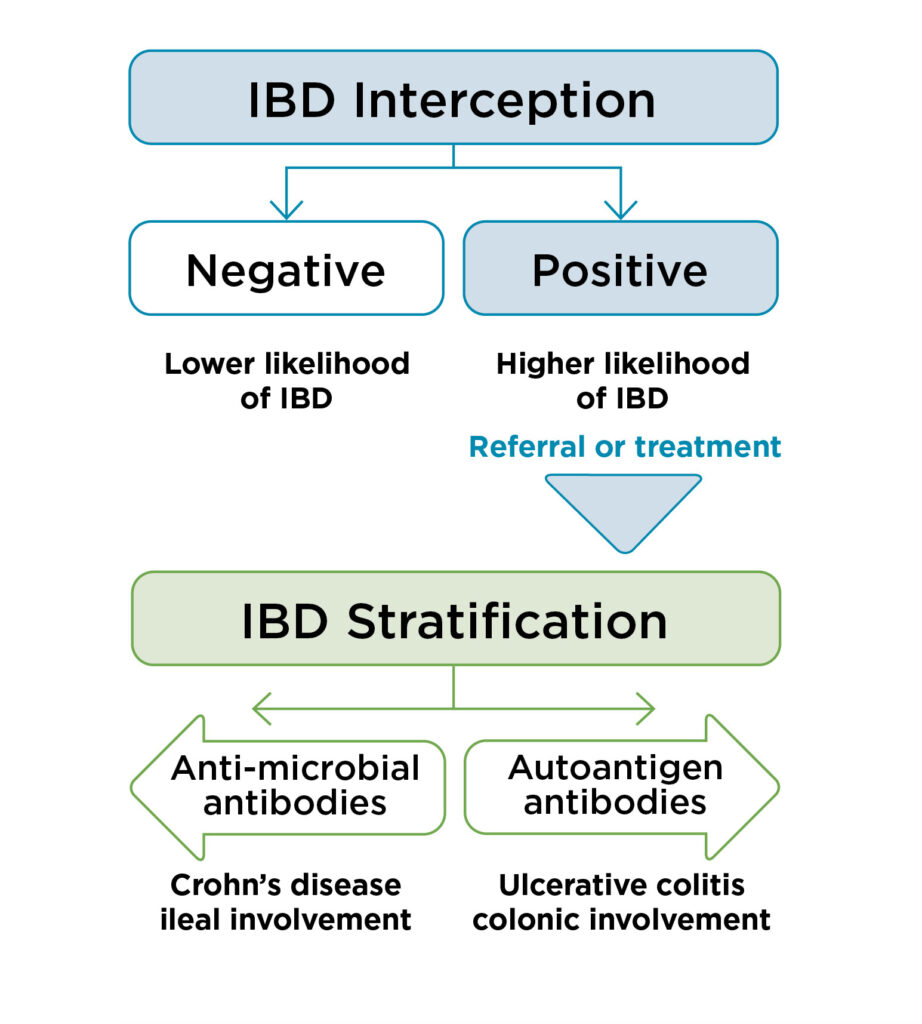
3. Antibody Result Summary
Antibody positivity is highly correlated with disease state.
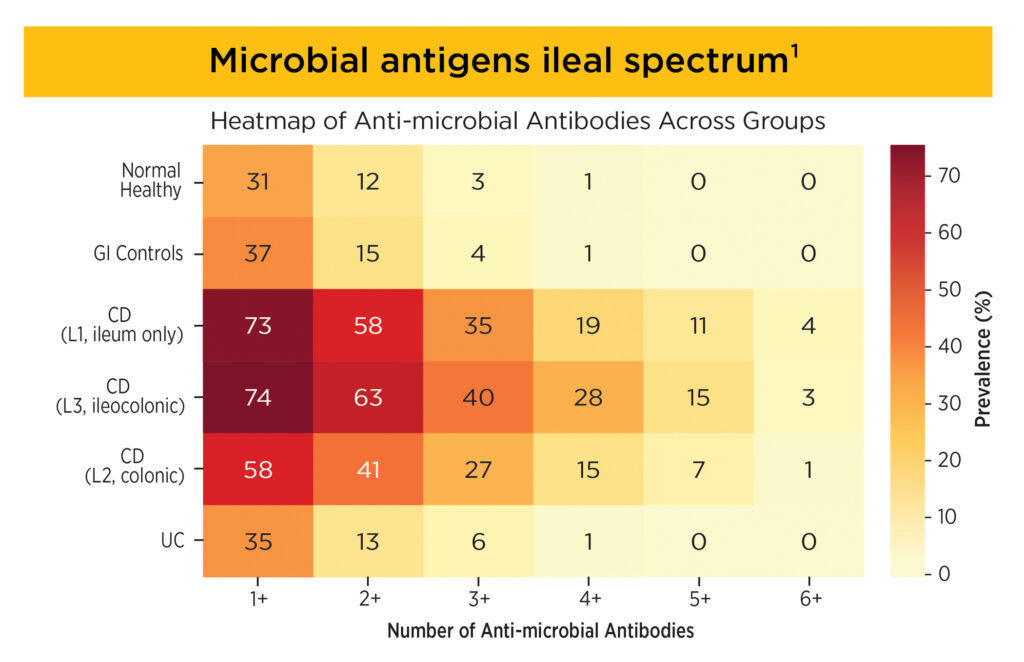
For Microbial Antigens: ≥3 positive antibodies offer high (>90%) specificity, making it a strong rule-in marker of CD.1

For Autoantigens: Triple positivity is rare, but highly specific for UC. Double positive also offers high specificity (89%) with a sensitivity of 56%.1
4. Insights on Antibodies Detected
The presence and concentration of specific antibodies can provide valuable information to help guide clinical management and risk stratification. Based on the antibodies detected in a patient’s sample, the report will include insights specific to the number and type of antibodies detected, as well as comments on clinical implications.
IBD Precis is part of the Prometheus continuum of testing solutions for IBD patients
IBD Precis is a laboratory-developed test that was developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA certified (05D0917432) and College of American Pathologists (CAP) accredited (6805501) clinical laboratory. As a laboratory developed test, it has not been cleared or approved by the US FDA. The test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. This material is provided for general information purposes only, as an educational service for healthcare providers. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Data on File.
- Choung RS et al. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2928–2937.e12.
- Mow WS et al. Gastroenterol. 2004;126(2):414-424.
- Targan SR et al. Gastroenterol. 2005 Jun;128(7):2020-2028.
- Schoepfer AM et al. Inflamm Bowel Dis. 2009 Sep;15(9):1358-1367.
- Torres J et al. Gastroenterol. 2020 Jul;159(1):96-104.
- Abreu et al. Clin Perspect Gastroenterol. 2001;4(3):155-164.
- Dubinsky MC et al. Clin Gastroenterol Hepatol. 2008 Oct;6(10):1105-1111.
- Fleshner P et al. Clin Gastroenterol Hepatol. 2008 May;6(5):561-568.
- Coukos JA et al. Dig Dis Sci. 2012 Jun;57(6):1544-1553.
- Kim JM et al. J Pediatr (Rio J). 2024 Mar-Apr;100(2):204-211.
- Andalucía C et al. Diagnostics (Basel). 2023 Dec;13(24):3682.
- Pertsinidou E et al. J Crohns Colitis. 2025 May;19(5):jjaf062.
- Livanos AE et al. Gastroenterol. 2023 Apr;164(4):619-629.
- Livanos A et al. J Crohns Colitis. OP28. 2025;19:i56-i58.
- Okabe M et al. J Gastroenterol. 2025 Jan;60(1):86-95.
- Nakanishi R et al. Inflamm Bowel Dis. 2025 Mar 3;31(3):777-785.
- Noor NM et al. Lancet Gastroenterol Hepatol. 2024 May;9(5):415-427.
- Choung RS et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1300-1310.
- Spencer EA et al. Inflamm Bowel Dis. 2018 May;24(6):1335-1343.
- Hamilton AL et al. J Gastroenterol Hepatol. 2017 Jun;32(6):1195-1203.
- Mahler M et al. Clin Chim Acta. 2013 Sep;424:267-73.
- Yanagida T et al. Intest Res. 2025 Apr. Epub ahead of print.









