Precision-Guided Dosing
![[LOGO] PredictrPK (R) [LOGO] PredictrPK (R)](/wp-content/uploads/2023/01/LOGO-PredictrPK-R.png)

Today’s biologic dosing challenges
Biologic therapy has revolutionized care for inflammatory bowel disease (IBD) and other immune-mediated inflammatory diseases (IMID). Anti-TNFs can be very effective for those who respond. Approximately 40% of IBD patients who start an anti-TNF do not respond1, up to 50% who initially respond lose response in the first year2 and over 50% will eventually require dose optimization.3
Newer biologics like anti-interleukins and anti-integrins are increasing in use, and patients face similar challenges with these drugs. Providers need ways to efficiently optimize biologic therapies since patients often have the most robust response to their first therapy.
Additionally, the cost of care is high, with the US spending over $70 billion annually on biologics related to treating these diseases.4

Why therapeutic drug monitoring isn’t enough
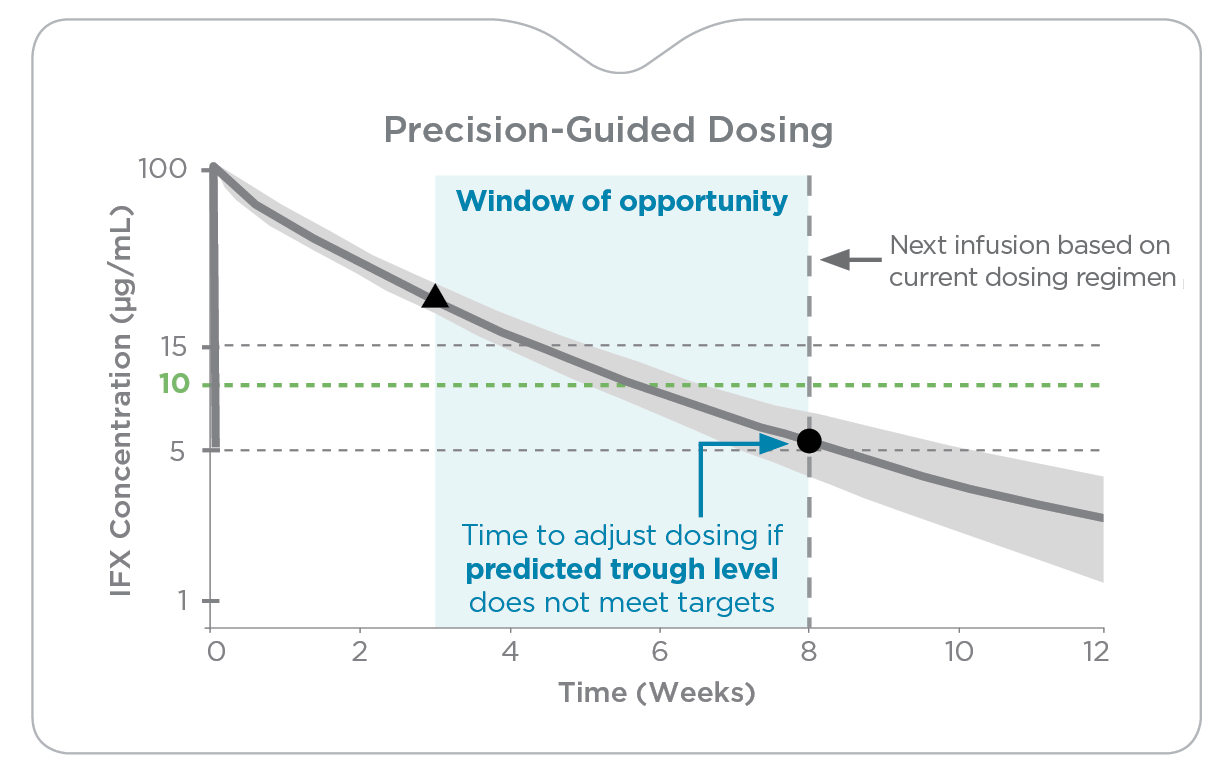
While therapeutic drug monitoring (TDM) has improved overall biologic success, there continue to be challenges in helping IBD patients reliably achieve established therapeutic and disease targets due to several factors:
- A narrow window of opportunity stemming from restrictions on sample collection due to lack of data on non-trough drug level targets
- Pharmacokinetic variables that contribute to differing responses amongst individuals5-6
- No objective measures of patient drug clearance
- Lack of predictive insights into potential dosing adjustments to achieve targets
Control the trajectory of therapeutic drug levels with precision-guided dosing
Precision-guided dosing with PredictrPK enables more accurate dose optimization by quantifying a patient’s drug clearance and considering significant pharmacokinetic (PK) variables to forecast their drug exposure over time.
This enables testing across a broader window so clinicians can:
- More precisely determine dose escalation where alternative dosing or interval adjustments may support recapturing and/or sustaining response and drug durability.
- Confidently evaluate feasibility of dose de-escalation.
- Quickly identify patients whose PK profiles suggest a switch in therapy may be required.
Unlike TDM, precision-guided dosing incorporates clearance, a PK factor that impacts drug exposure and overall drug bioavailability by measuring the volume of drug-containing plasma removed from the body each day. Changes in clearance may precede changes in drug levels and may be an early predictor of therapeutic response.6
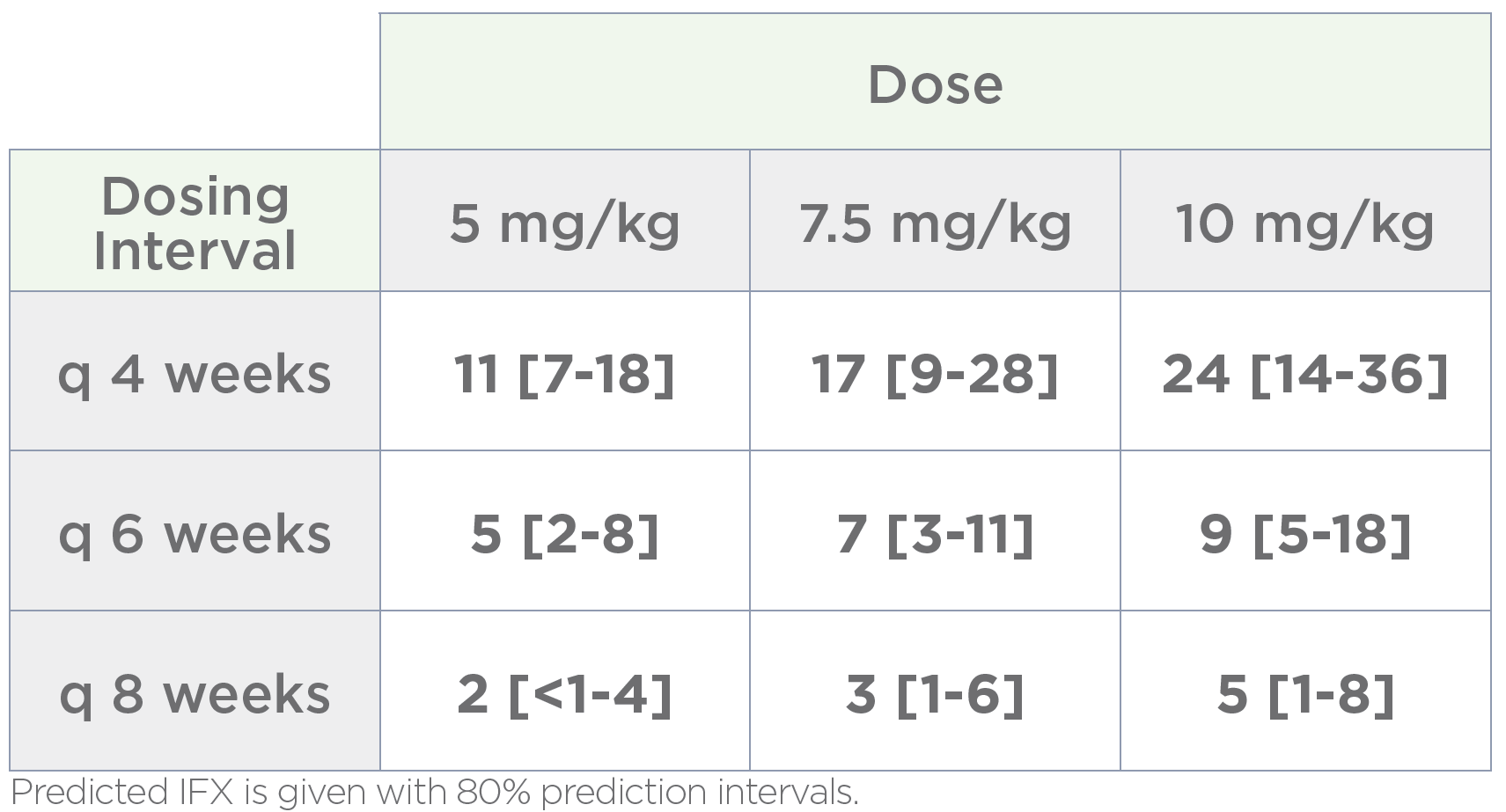
Patient-specific dosing guidance
Bayesian forecasting is applied to each patient’s pharmacokinetic profile to develop a patient-specific current and alternative dosing schedule, providing clear guidance to help patients achieve target drug levels.
This objective evidence enables providers to have productive dialog with patients about therapeutic options and offers payers data to inform medical authorizations.
PredictrPK is currently available for the following biologics and their respective biosimilars:
PredictrPK is part of the Prometheus continuum of care for IBD patients
PredictrPK tests are laboratory-developed tests that were developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and are performed exclusively in our high complexity CLIA-certified (05D0917432) and College of American Pathologists-accredited (6805501) clinical laboratory. As laboratory developed tests, they have not been cleared or approved by the US FDA. These tests may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. PredictrPK IFX Maintenance and IFX Induction tests are validated for IBD patients receiving intravenous IFX. PredictrPK ADA is validated for IBD patients ≥12 years old. PredictrPK VDZ (intravenous only) and UST Maintenance are validated for IBD patients ≥18 years. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
-
- Syed et al. Crohn’s & Colitis 360. 2020;2:otaa050.
- Ben-Horin et al. Autoimmun Rev. 2014; 13:24-30.
- O’Donnell et al. J Crohns Colitis. 2015;830–836.
- Aitken et al. IQVIA Institute for Human Data Science. April 2018.
- Vermeire et al. Clin Gastroenterol Hepatol. 2020;18:1291–1299.
- Polasket T and R Peck. Clin Pharm & Ther. 2024;116(3):602-12.









