Precision-Guided Dosing


PredictrPK provides evidence-based insights to precisely optimize adalimumab therapy
Prolonged exposure to subtherapeutic adalimumab (ADA) concentrations can impact treatment efficacy, leading to disease progression and potentially avoidable hospitalization or surgery. With wide interpatient variability in therapeutic response and biologic persistence, simply measuring drug levels and antidrug antibody status with traditional therapeutic drug monitoring isn’t enough to accurately predict the trajectory of treatment.
PredictrPK® ADA assesses the unique pharmacokinetic profile of your inflammatory bowel disease (IBD) patient to guide optimal timing and administration of ADA during maintenance.
Higher ADA levels and lower ADA clearance are associated with improved clinical outcomes1
ADA concentrations >10 μg/mL associated with a 4.5-, 2.1- and 3.2-fold increased likelihood of clinical remission, endoscopic remission and fecal calprotectin <100 μg/g, respectively.
ADA clearance <0.318 L/day associated with a 6.5-, 2.5- and 4.1-fold increased likelihood of clinical remission, endoscopic remission and fecal calprotectin <100 μg/g, respectively.
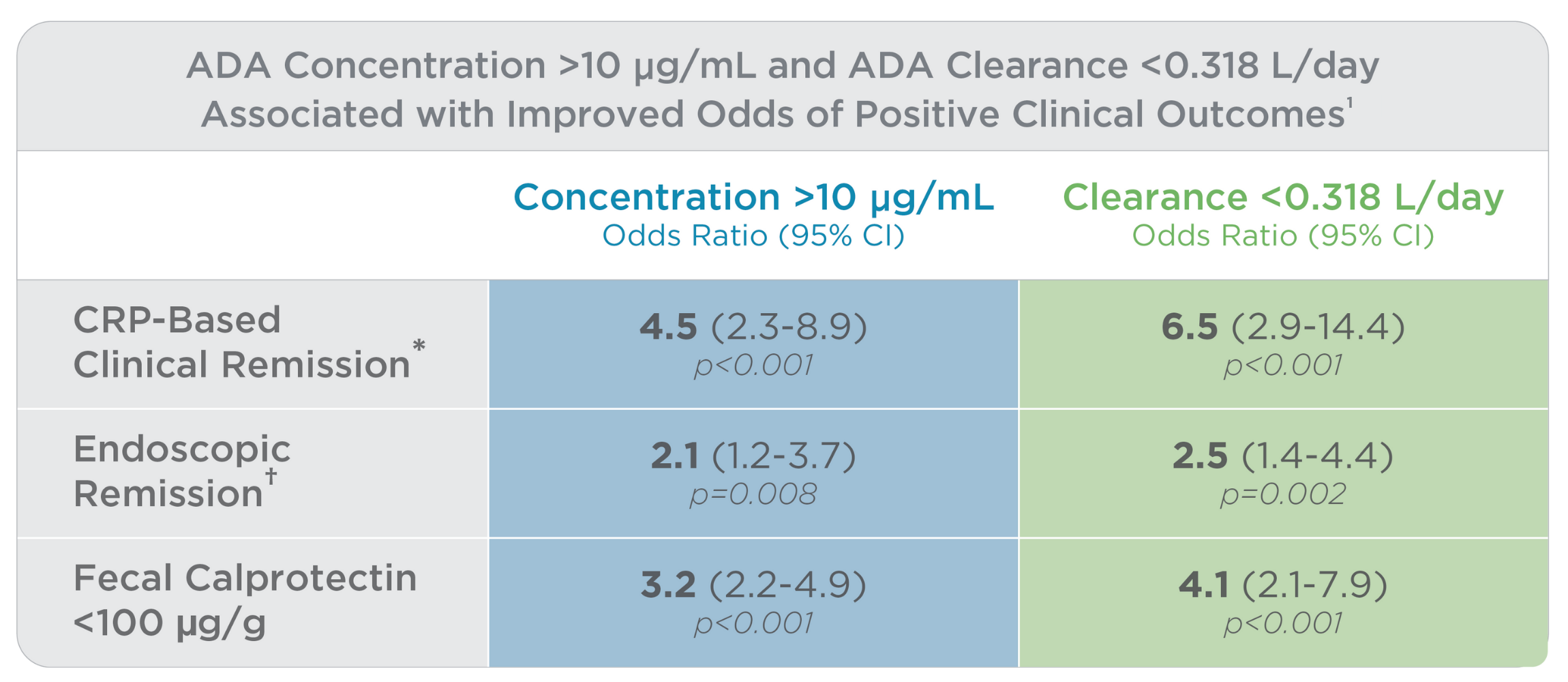
*Clinical remission: CRP <3 mg/L, HBI <5 and CDAI <150 points; ᶧEndoscopic remission: SES-CD <3 points; Normal fecal calprotectin: <100 μg/g
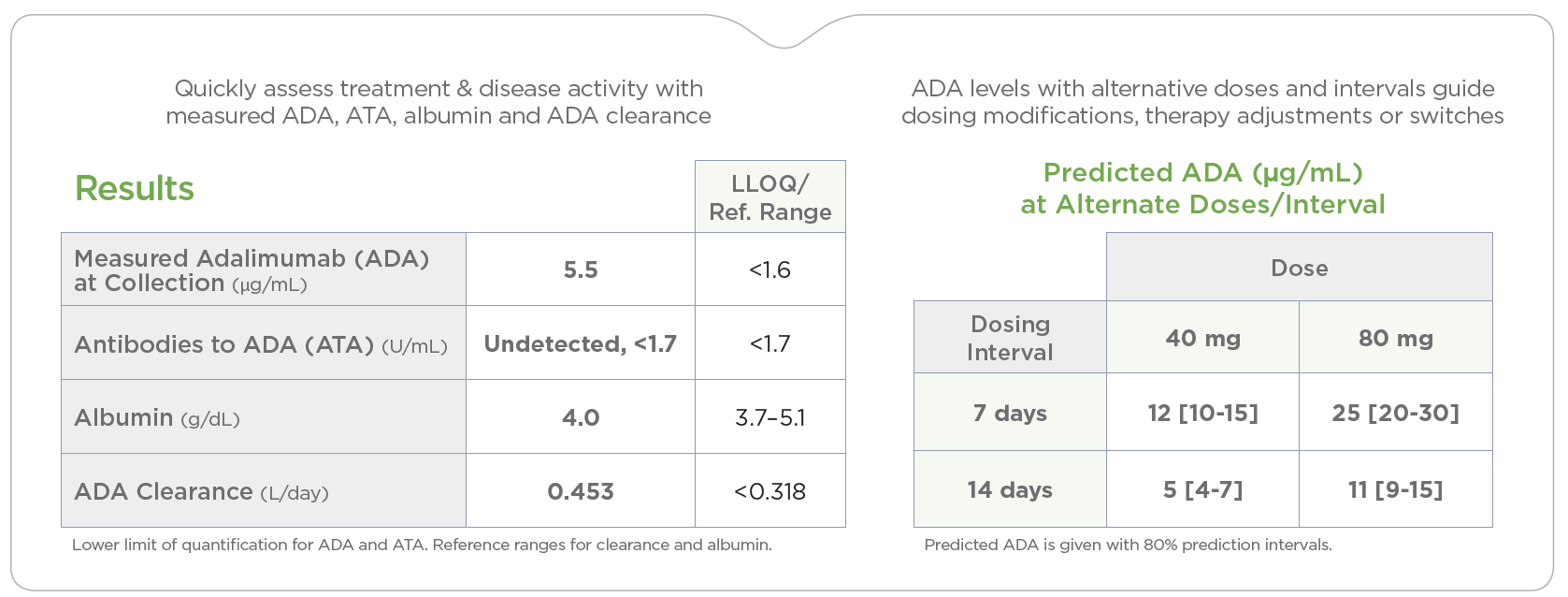
PredictrPK ADA Maintenance provides personalized, data-driven pharmacokinetic (PK) dosing guidance and individual drug clearance to efficiently optimize ADA therapy through timely treatment adjustments, supporting more effective and cost-efficient care.
By analyzing the factors known to influence drug response and durability, PredictrPK ADA provides tailored ADA dosing guidance in support of drug persistence, sustained clinical remission and improved outcomes.
The EMPOWER ADA prospective clinical experience study examined the clinical utility of PredictrPK ADA for optimizing ADA therapy in patients with IBD and found2:
- It enabled early and more precise optimization of ADA therapy by predicting future trough levels at alternative doses and intervals at any time during the therapy cycle.
- 53% of patients demonstrated accelerated clearance, which puts them at risk of inadequate drug exposure or antidrug antibody development.
Benefits of PredictrPK ADA to HCPs surveyed2
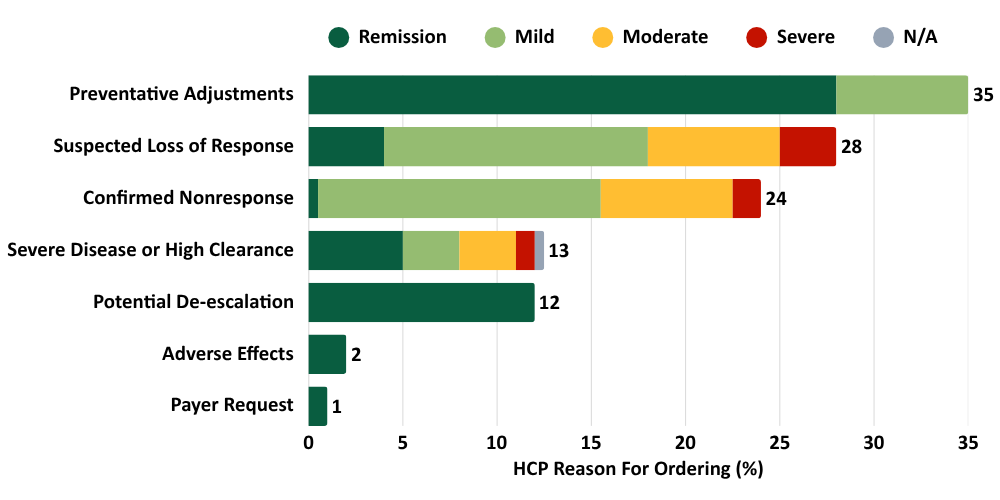
Note: HCPs could select more than one reason for ordering, so total exceeds 100%
PredictrPK ADA was developed and validated using 1000+ ADA cycles from 350+ adult and pediatric Crohn’s disease patients.15
Precision-guided dosing for ADA increases the likelihood of achieving therapeutic targets, recapturing clinical response and shortening the time to remission.1-6,15
PredictrPK is currently available for the following biologics and their respective biosimilars:
PredictrPK is part of the Prometheus continuum of care for IBD patients
PredictrPK tests are laboratory-developed tests that were developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and are performed exclusively in our high complexity CLIA-certified (05D0917432) and College of American Pathologists-accredited (6805501) clinical laboratory. As laboratory developed tests, they have not been cleared or approved by the US FDA. These tests may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. PredictrPK ADA is validated for IBD patients ≥12 years old. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Wright et al. J Crohns Colitis. 2024;18:212-222.
- Hanauer SB et al. Pharmaceutics. 2025; 17(4):428.
- Vande Casteele et al. J Crohns Colitis. 2019; 13:1248–1256.
- Papamichael et al. J Crohns Colitis. 2019; 13:976-981.
- Vermeire et al. Clin Gastroenterol Hepatol. 2020; 18:1291–1299.
- Ziring D et al. Gastroenterol. 2025;S-1535-S-1536.
- Cheifetz et al. Am J Gastroenterol. 2021; 116:2014-2025.
- Vaugh B. J Clin Med. 2021; 10:4990.
- Karmiris et al. Gastroenterol. 2009; 137:1628–1640.
- Kennedy et al. Lancet Gastroenterol Hepatol. 2019; 4:341-353.
- Juncadella et al. Dig Dis Sci. 2018; 63:3067-3073.
- Mantzaris et al. Crohns Colitis 360. 2021; 3:otab064.
- Baert et al. Gut. 2016; 65:1126-1131.
- Ungar et al. Am J Gastroenterol. 2018; 113:890-898.
- Data on file.








