Precision-Guided Dosing


PredictrPK IFX provides forward-looking insights to guide infliximab dosing
Roughly two in five patients fail to respond to infliximab (IFX) during induction2 and approximately 50% of responders require additional dose optimization during maintenance.3
PredictrPK® IFX assesses the unique pharmacokinetic profile of your inflammatory bowel disease (IBD) patient to guide optimal timing and administration of IFX infusions during induction and maintenance.

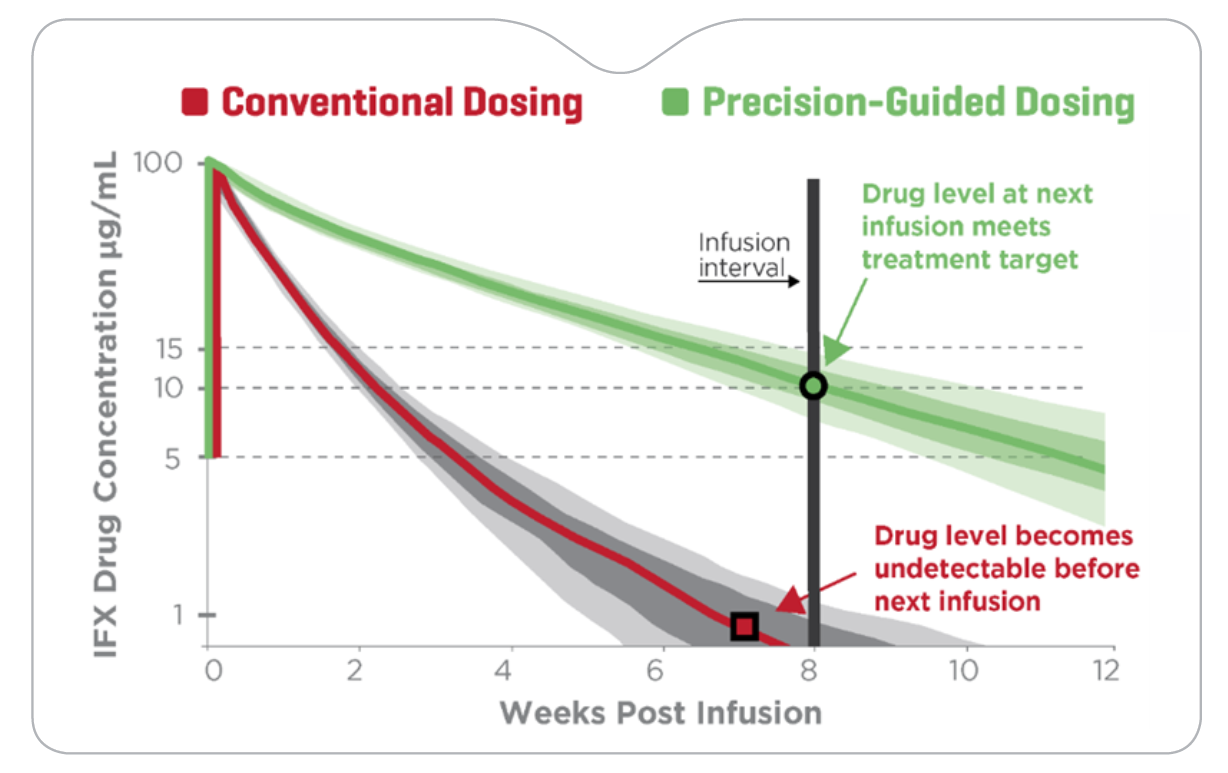
PredictrPK IFX Induction guides optimal timing of dose 4, which was found to be key to week 52 drug durability and corticosteroid-free remission.
The PRECISION IFX prospective trial examined the impact of precision-guided induction dosing on patient outcomes at one year and found4:
- Targeting dose 4 IFX trough levels ≥10 μg/mL, the majority of patients required accelerated administration of infusion 4.
- 73% of patients who received infusion 4 were still on IFX at week 52, a significant improvement compared to previous studies, where only approximately 30% remained on IFX beyond one year.
- 97% of patients on IFX at week 52 were in steroid-free clinical remission.
A retrospective analysis of this trial found low clearance and optimized IFX at infusion 4 resulted in5:
- Shorter time to remission (3.1-fold faster at ~147 days).
- Higher likelihood of sustained remission (5-times more likely).
- Higher remission rates at 1-year (95% vs. 44%).
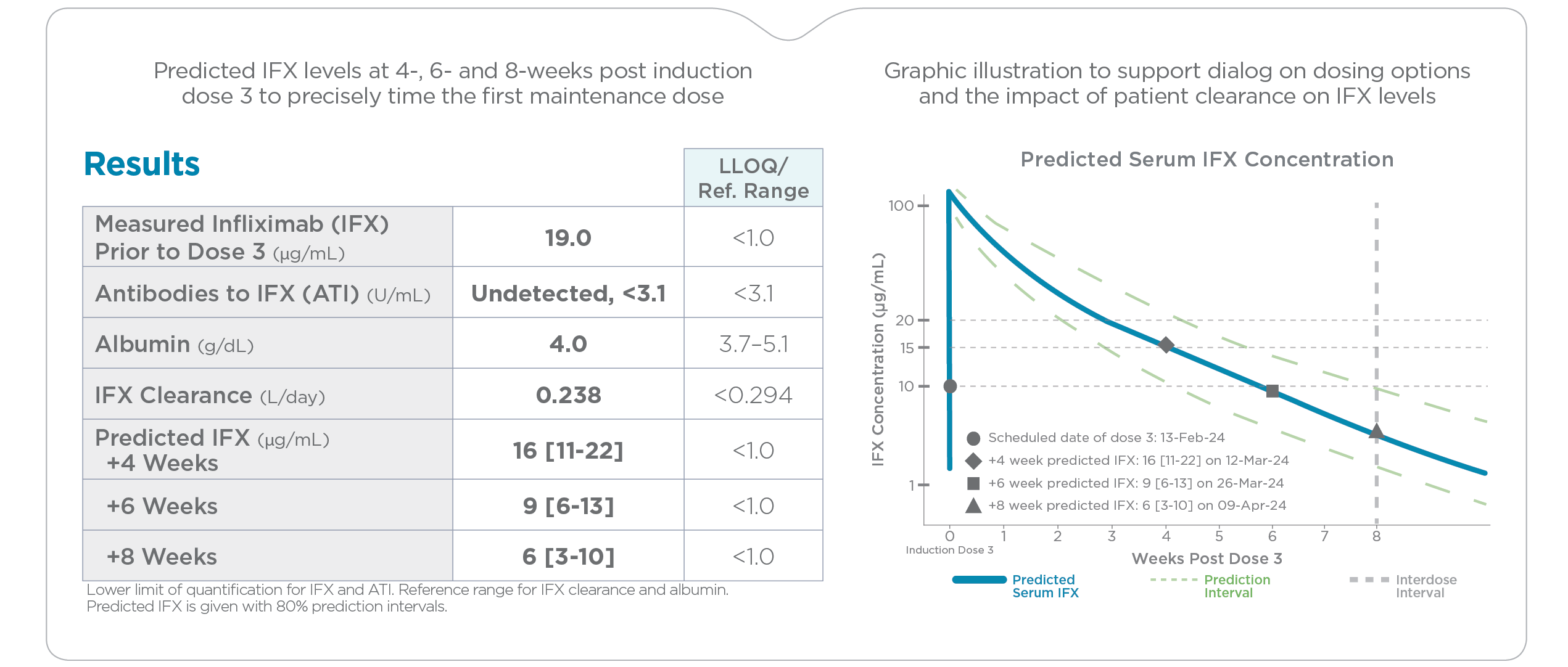
PredictrPK IFX Induction was developed with 300+ IFX cycles [308 patients | 81% CD | 49% female | 55% pediatric]1
PredictrPK IFX Maintenance provides personalized, data-driven pharmacokinetic (PK) dosing guidance and individual drug clearance to increase the likelihood of achieving treatment targets.
PredictrPK IFX testing during maintenance quickly assesses a patient’s pharmacokinetic (PK) profile, including clearance, to facilitate individualized precision-dosing of IFX. The test offers actionable insights with projected serum IFX concentrations based on the current dosing regimen as well as with alternative dosing options.
A retrospective, multi-center chart review compared pre-PredictrPK test and post-PredictrPK outcomes and found6:
- Improvement in disease classification.
- Significant reduction in hospitalizations and length of hospitalizations.
- 12-month cost savings of ~$4,461 per patient.
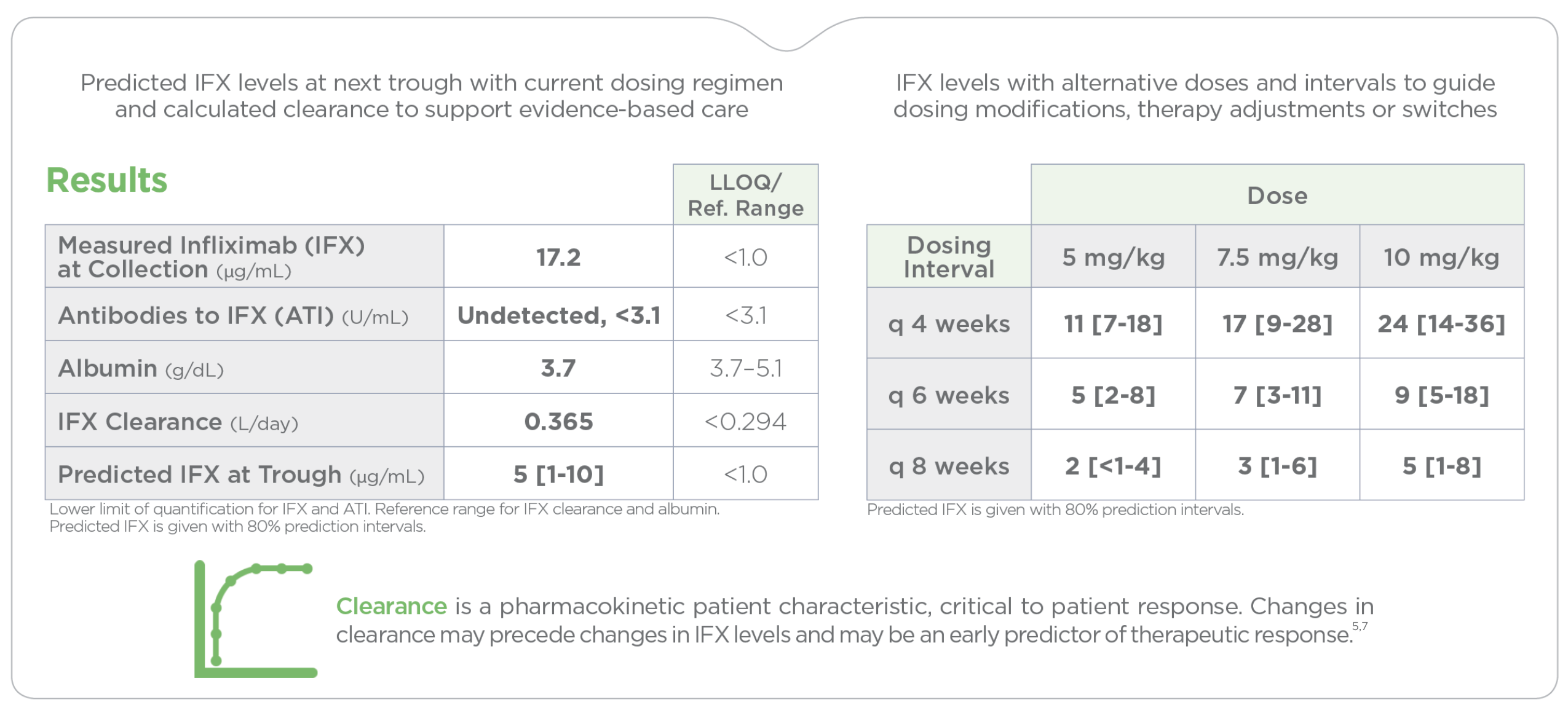
PredictrPK IFX Maintenance was developed with 1,000+ IFX cycles [343 patients | 83% CD | 49% female | 50% pediatric]1,8
Precision-guided dosing for IFX increases the likelihood of achieving therapeutic targets, recapturing clinical response and shortening the time to remission.1,4-9
PredictrPK is currently available for the following biologics and their respective biosimilars:
PredictrPK is part of the Prometheus continuum of care for IBD patients
PredictrPK tests are laboratory-developed tests that were developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and are performed exclusively in our high complexity CLIA-certified (05D0917432) and College of American Pathologists-accredited (6805501) clinical laboratory. As laboratory developed tests, they have not been cleared or approved by the US FDA. These tests may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. PredictrPK IFX Maintenance and IFX Induction tests are validated for IBD patients receiving intravenous IFX. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
-
- PredictrPK dossier. Data on file.
- Syed et al. Crohn’s & Colitis 360. 2020;2:otaa050.
- O’Donnell et al. J Crohns Colitis. 2015;830–836.
- Dubinsky et al. Inflamm Bowel Dis. 2022;28:1375-1385.
- Dubinsky et al. Pharmaceutics. 2023;15:2408.
- Arai et al. Crohn’s & Colitis 360. 2025 Jul.
- Deyhim et al. Clin Med. 2023 Nov 16;12:7132.
- Primas et al. J Clin Med. 2022; 11:3316.
- Abraham et al. Am J Manag Care. 2023;29:S227-S235.
- Ben-Horin S et al. Autoimmun Rev. 2014;13(1)24-30.








