Precision-Guided Dosing


PredictrPK VDZ incorporates clearance data to help you tailor vedolizumab therapy
Without personalized dosing guided by objective, patient-specific pharmacokinetic data such as clearance, patients face risks such as suboptimal drug exposure, loss of response and avoidable complications.1-3
PredictrPK® VDZ assesses the unique pharmacokinetic profile of your inflammatory bowel disease (IBD) patient to guide optimal timing and administration of vedolizumab (VDZ) during maintenance.
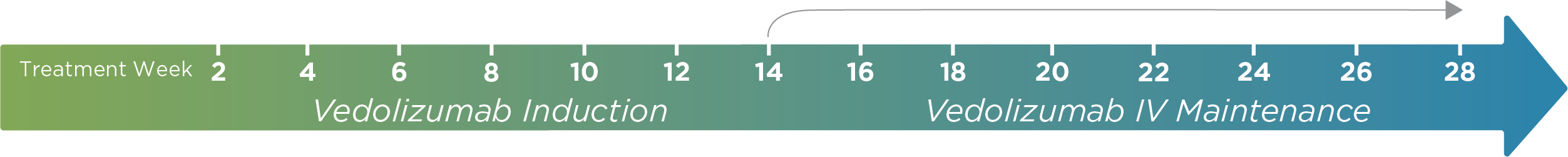
Sample collection: ≤3 days prior to week 14 or, after ≥14 weeks of VDZ therapy, anytime ≥20 days after any maintenance infusion, up to and including at trough.
Higher VDZ clearance negatively impacts patient outcomes
A critical and overlooked pharmacokinetic variable, clearance impacts clinical response. Standard dosing may fall short as patients with elevated drug clearance may not reach therapeutic targets.
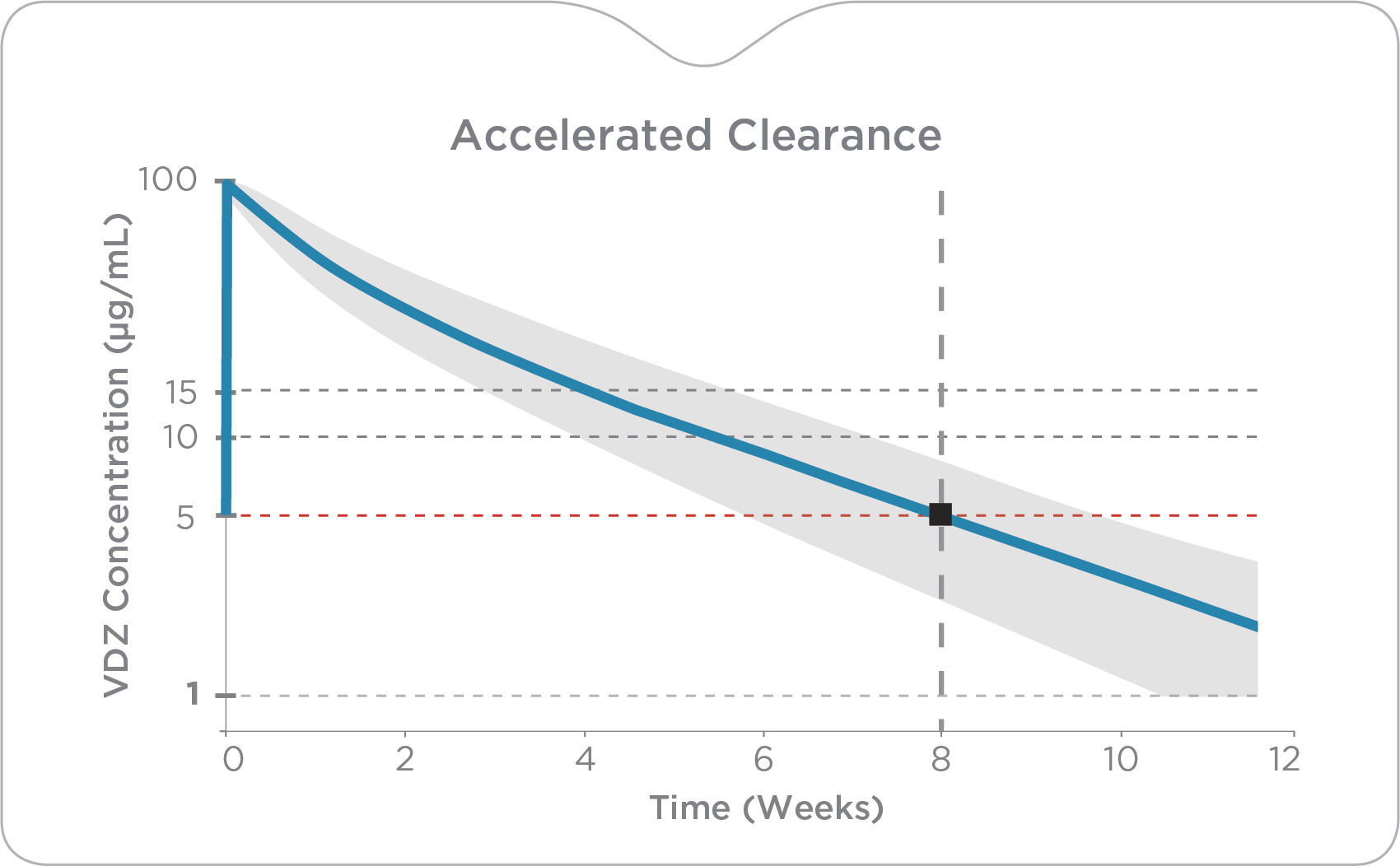
Accelerated VDZ Clearance
Accelerated drug clearance increases the likelihood of inadequate drug exposure and subsequent endoscopic nonresponse, increased steroid use, hospitalizations and poor long-term outcomes. Patients with high VDZ clearance (≥0.23) show dramatically lower clinical response (26.6%) and remission (5.9%) rates at week 14.4

Non-Accelerated VDZ Clearance
Conversely, by week 6, patients with low clearance (<0.14 L/day) had a higher likelihood of achieving response (65.5%) and remission (35.7%) as compared to those with higher clearance.4
PredictrPK VDZ Maintenance is a clinical support tool that personalizes VDZ maintenance dosing, enabling fine-tuning of 300 mg infusion (IV) frequency based on predicted trough levels and patient-specific clearance.
Clearance offers clinical evidence to help tailor dosing in support of optimized outcomes.
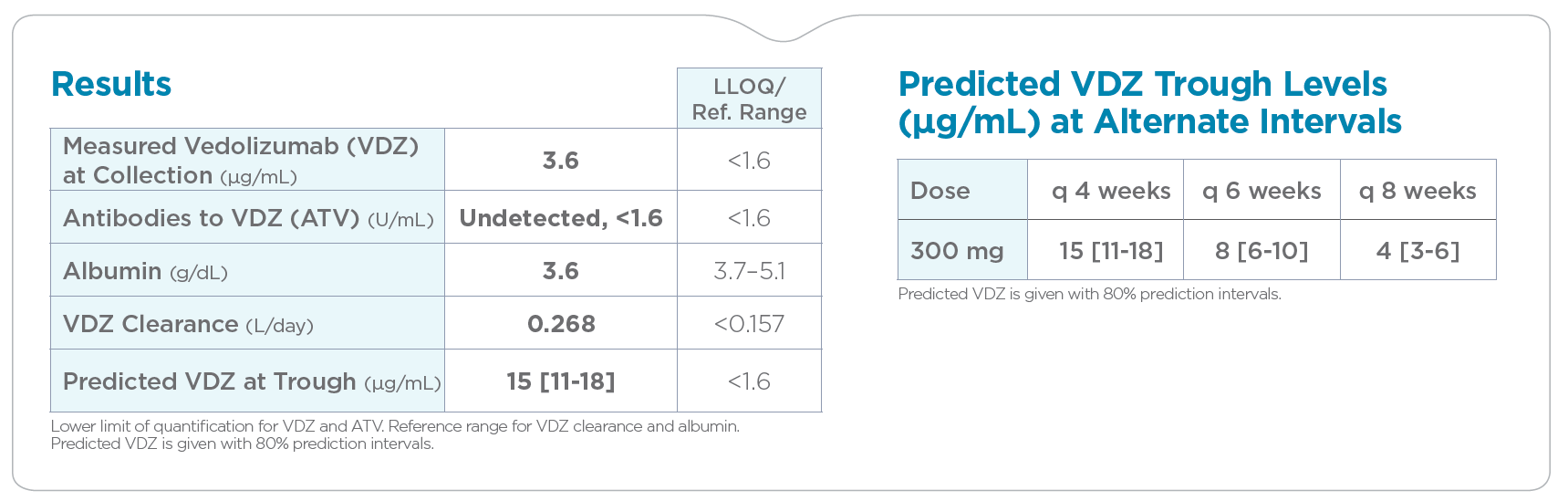
PredictrPK VDZ was developed and validated using 300+ VDZ cycles from 50 adult IBD patients.5
Precision-guided dosing for VDZ increases the likelihood of achieving therapeutic targets, recapturing clinical response and shortening the time to remission.1-5
PredictrPK is currently available for the following biologics and their respective biosimilars:
PredictrPK is part of the Prometheus continuum of care for IBD patients
PredictrPK tests are laboratory-developed tests that were developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and are performed exclusively in our high complexity CLIA-certified (05D0917432) and College of American Pathologists-accredited (6805501) clinical laboratory. As laboratory developed tests, they have not been cleared or approved by the US FDA. These tests may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. PredictrPK VDZ (intravenous only) Maintenance is validated for IBD patients ≥18 years. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.









