Inflammatory Bowel Disease

Determine risk of anti-TNF immunogenicity before initiating anti-TNF therapy in IBD patients.
RiskImmune® is a laboratory-developed test that may help identify patients at greater risk of anti-TNF antibody (ADAb) formation and could aid your therapeutic decision making.
This blood-based genetic test identifies if an IBD patient is a variant carrier of HLADQA1*05 (rs2097432), a single nucleotide polymorphism that has been associated with:
- A 2-fold increased risk of developing antibodies to infliximab or antibodies to adalimumab1
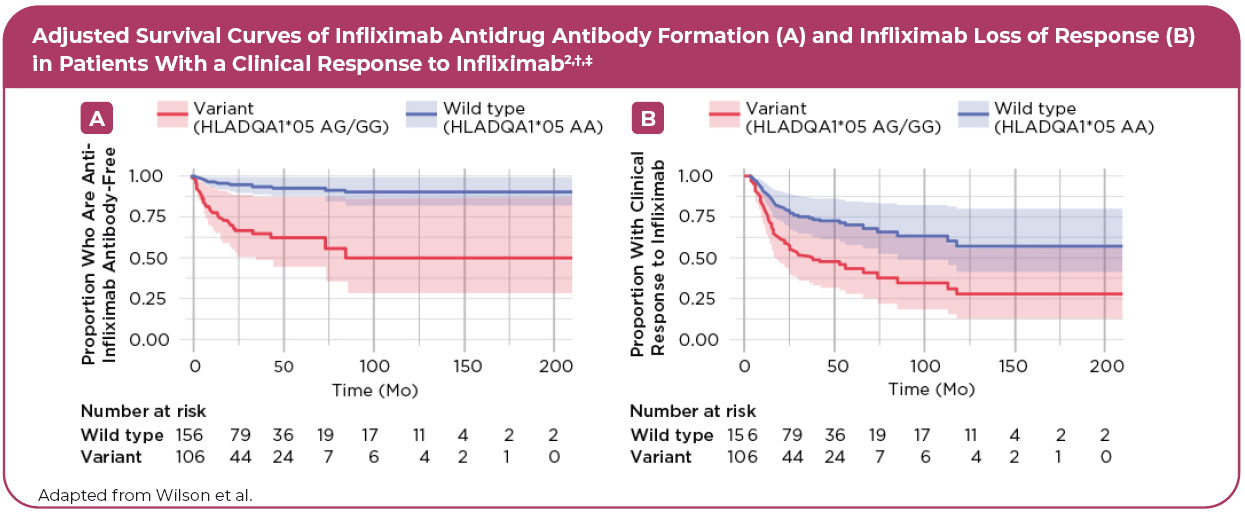
• Regardless of concomitant immunomodulator use and the drug type1,* - A 7-fold increased risk of developing antibodies to infliximab2,†
• Associated with a 2-fold increase in loss of response to infliximab2,†
• Variant carriers were 2 times more likely to discontinue infliximab therapy2,†
RiskImmune can help you optimize IBD patient care from the start
Variant carriers2,*:
- Had a significantly increased risk of infliximab ADAb formation versus nonvariant carriers—even when adjusted for age, sex, weight, dose, and coimmunosuppression
- Were at an increased risk of infliximab loss of response and had a higher rate of infliximab discontinuation versus nonvariant carriers
Get ahead of the impact of immunogenicity with RiskImmune.
- Helps you understand an IBD patient’s risk of developing immunogenicity to infliximab or adalimumab
- Aids in being proactive with treatment choices and therapeutic drug monitoring

TNF = tumor necrosis factor; IBD = inflammatory bowel disease.
* Based on the Personalising Anti-TNF Therapy in Crohn’s Disease (PANTS) clinical study, which included a discovery cohort of 1240 biologic-naïve patients with Crohn’s disease starting infliximab or adalimumab and a replication cohort of 178 patients with IBD. The PANTS study only included subjects of European descent, 40% of whom carry the risk allele. Immunogenicity was defined as an antidrug antibody concentration of ≥ 10 AU/mL, irrespective of drug level, at 1 or more time points.
†Based on a separate retrospective study of 262 IBD patients on infliximab therapy of which 40% identified as variant carriers.
‡According to HLADQA1*05>G genotype from a Cox proportional hazard model. Data were adjusted for age, sex, weight, infliximab dose, and coimmunosuppression with an immunomodulator (azathioprine or methotrexate). The 95% confidence intervals are represented by blue (HLADQA1*05 wild type) and red (HLADQA1*05 variant) shading.
References
- Sazonovs A, Kennedy NA, Moutsianas L, et al; PANTS Consortium. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology. 2020;158(1):189-199.
- Wilson A, Peel C, Wang Q, Pananos AD, Kim RB. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51(3):356-363.
RiskImmune is a laboratory-developed test that was developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA certified (05D0917432) and College of American Pathologists (CAP) accredited (6805501) clinical laboratory. As a laboratory developed test, it has not been cleared or approved by the US FDA. The test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. Prometheus and RiskImmune are registered trademarks of Prometheus Laboratories Inc, San Diego, California. All other trademarks or service marks are the property of their respective owners. This material is provided for general information purposes only, as an educational service for healthcare providers. It is not intended as a substitute for medical advice and/or consultation with a physician.
