Therapeutic Drug Monitoring

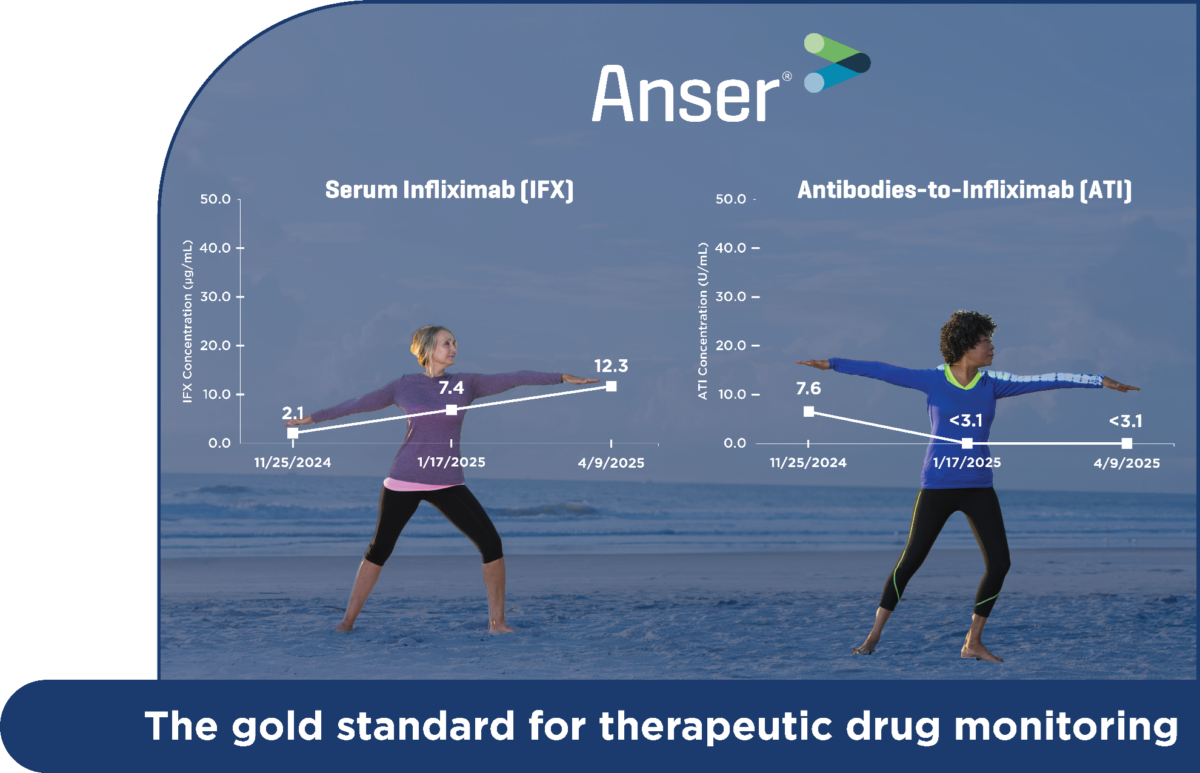
Anser® therapeutic drug monitoring (TDM) tests support biologic optimization and guide clinical decisions for patients treated with:
- Infliximab (Anser IFX)*†
- Adalimumab (Anser ADA)*
- Vedolizumab (Anser VDZ)
- Ustekinumab (Anser UST)*
- Risankizumab (Anser RZB)
*And their respective biosimilars
†Including subcutaneous infliximab
Advantages of the Anser Platform:
- Trusted by providers: Nearly 480,000 tests performed for more than 220,000 individual patients, helping inform treatment decisions for over 10 years.
- Simultaneously quantifies drug and anti-drug antibody levels in serum using a proprietary drug-tolerant homogeneous mobility shift assay (HMSA) methods without interference from detectable drug – a limitation of many solid-phase assays like ELISA.1,2
- Anser IFX and Anser ADA HMSA platforms have been validated against World Health Organization (WHO) IFX and ADA standards.3,4†
- Trusted by researchers: Anser testing relied upon for accurate data in more than 100 published clinical studies.
†WHO standards are not available for VDZ, UST or RZB
Anser tests are laboratory-developed tests that were developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and are performed exclusively in our high complexity CLIA-certified (05D0917432) and College of American Pathologists-accredited (6805501) clinical laboratory. As laboratory a developed tests, they have not been cleared or approved by the US FDA. These tests may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References

