Precision-Guided Dosing

![[Page Header] ADAm 20230705 [Page Header] ADAm 20230705](https://prometheuslabs.com/wp-content/uploads/2023/07/Page-Header-ADAm_20230705.png)
Unoptimized therapy puts an unnecessary strain on patients, healthcare resources and risks treatment failure, resulting in a switch to more expensive and often less effective therapies.1-5
![[Empiric vs PGD] ADA (Combined) 20230705+JG (caption only) [Empiric vs PGD] ADA (Combined) 20230705+JG (caption only)](https://prometheuslabs.com/wp-content/uploads/2023/07/Empiric-vs-PGD-ADA-Combined_20230705JG-caption-only.png)
Prolonged exposure to subtherapeutic adalimumab (ADA) concentrations can impact treatment efficacy leading to harmful disease progression and potentially avoidable hospitalization or surgery. PredictrPK® ADA provides objective, individualized, pharmacokinetic dosing guidance in support of more efficient and cost-effective care.
![[Bugs] Horizontal (large) 20230622 [Bugs] Horizontal (large) 20230622](https://prometheuslabs.com/wp-content/uploads/2023/07/Bugs-Horizontal-large_20230622.png)
Every patient deserves to focus on life, not their disease. Let their unique pharmacokinetic profile
help guide treatment to position adalimumab therapy for durable and sustained response
Expediently fine-tune dosing through dose escalation, de-escalation and other treatment modifications.
![[Stylized Results] 20230620 [Stylized Results] 20230620](https://prometheuslabs.com/wp-content/uploads/2023/07/Stylized-Results_20230620.png)
By analyzing pharmacokinetic factors known to influence drug response and durability,6, 16 PredictrPK ADA provides tailored ADA dosing guidance in support of drug persistence, sustained clinical remission and improved outcomes.
PredictrPK ADA addresses the unmet need for individualized optimization of ADA
and the wide interpatient variability in initial therapeutic response and biologic persistence
Developed and validated using 800+ ADA cycles from 200+ adult Crohn’s disease patients17
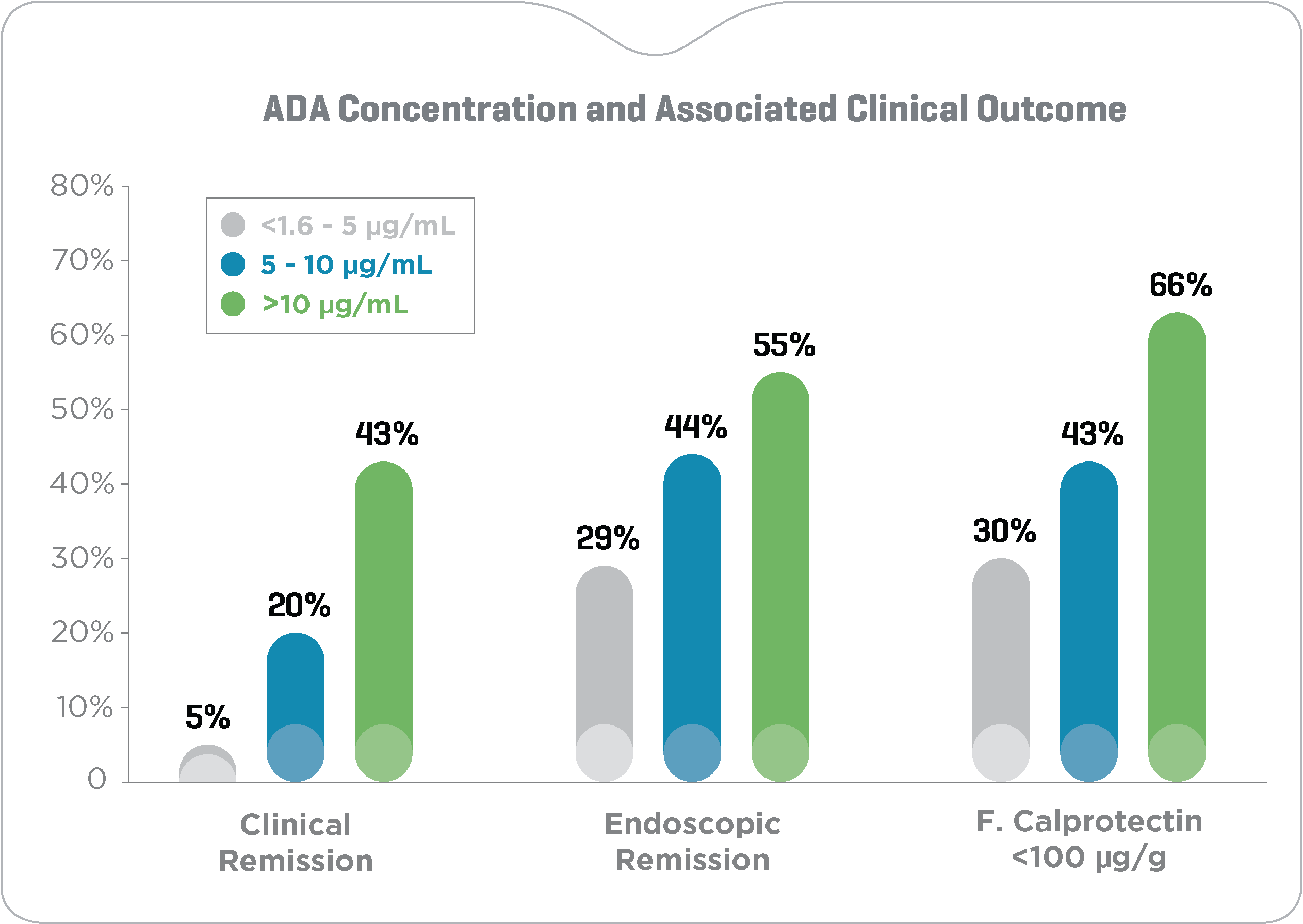
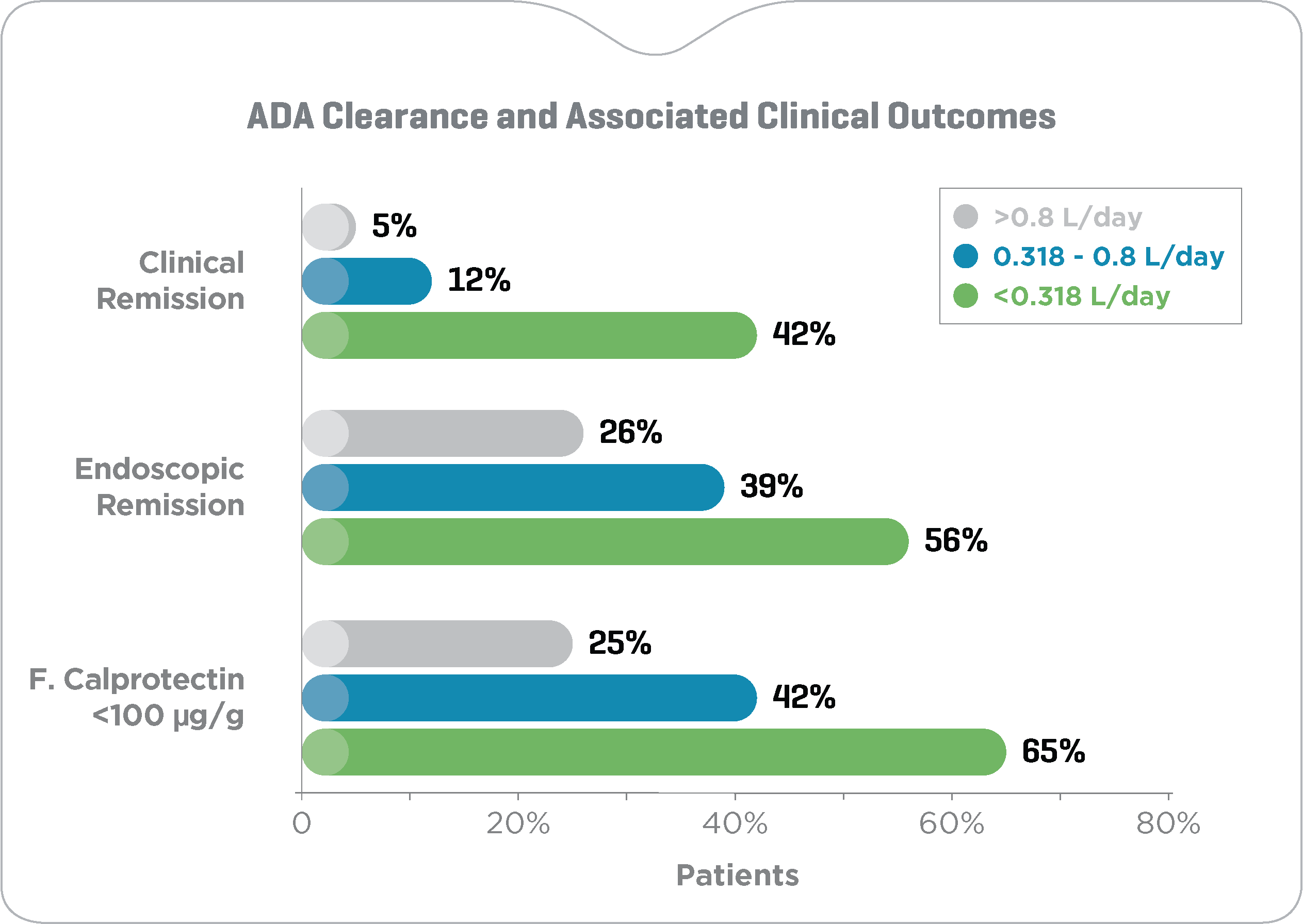
Higher ADA levels and lower ADA clearance during maintenance are associated with improved clinical outcomes
Maintenance ADA concentrations >10 μg/mL (fig. 1) and ADA clearance <0.318 L/day (fig. 2) associated with a 2- to 4-fold and 3- to 6-fold
increased likelihood of clinical and endoscopic remission and normalization of fecal calprotectin in adult Crohn’s disease patients, respectively.17
Clinical remission: CRP <3 mg/L, HBI <5 and CDAI <150 points; Endoscopic remission: SES-CD <3 points; Normal fecal calprotectin: <100 μg/g
Powerful performance supporting the clinical utility of precision-guided dosing with PredictrPK ADA
*PredictrPK ADA was validated in a cohort of adult Crohn’s disease patients receiving ADA therapy including patients on combination therapy with an immunomodulator or another therapeutic agent. PredictrPK ADA can be utilized during steady-state maintenance, after 8 weeks of continuous, on-time, ADA therapy. Serum samples can be collected anytime within the prescribed dosing interval.
References
- Pariente et al. Inflamm Bowel Dis. 2011; 17:1415-22.
- Cheifetz et al. Am J Gastroenterol. 2021; 116:2014-2025.
- Vermeire et al. Clin Gastroenterol Hepatol. 2020; 18:1291–1299.
- Eser et al. J Clin Pharmacol. 2018; 58:790-802.
- Vaugh B. J Clin Med. 2021; 10:4990.
- Ordas I et al. Clin Pharmacol Ther. 2012; 91:635-646.
- Karmiris et al. Gastroenterol. 2009; 137:1628–1640.
- Kennedy et al. Lancet Gastroenterol Hepatol. 2019; 4:341-353.
- Juncadella et al. Dig Dis Sci. 2018; 63:3067-3073.
- Ben-Horin et al. Autoimmun Rev. 2014; 13:24-30.
- Mantzaris et al. Crohns Colitis 360. 2021; 3:otab064.
- Baert et al. Gut. 2016; 65:1126-1131.
- Vande Casteele et al. J Crohns Colitis. 2019; 13:1248–1256.
- Ungar et al. Am J Gastroenterol. 2018; 113:890-898.
- Papamichael et al. J Crohns Colitis. 2019; 13:976-981.
- Dubinsky et al. Inflamm Bowel Dis. 2022; 28:1375-1385.
- Primas et al. J Clin Med. 2022; 11:3316.
- Data on Prometheus Laboratories.



