Inflammatory Bowel Disease

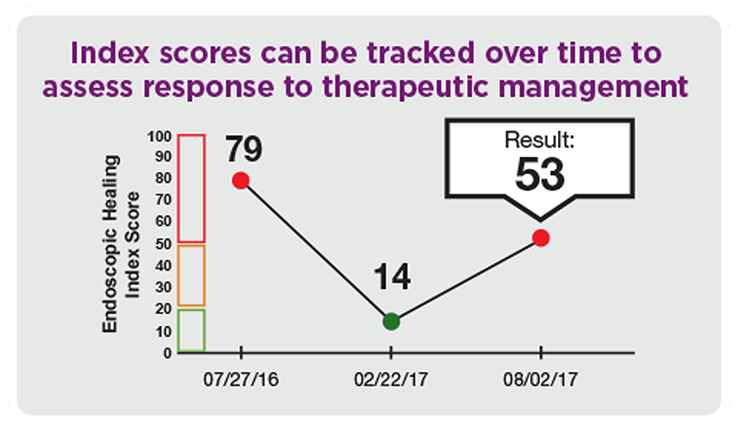
Measure & monitor mucosal healing changes over time
Improvement in mucosal damage provides an objective measure of therapeutic response in inflammatory bowel disease (IBD) management.1,2 However, current assessment methods may not provide needed information, particularly when evaluating disease activity in difficult-to-reach locations to assess ideal disease.1,3-4
Monitr® is a laboratory-developed test that evaluates multiple markers of mucosal damage and repair processes, regardless of disease location.4 It applies a proprietary algorithm to 13 biomarkers to produce a quantitative Endoscopic Healing Index (EHI) Score — ranging from 0 to 100 — to aid in distinguishing endoscopic remission from active disease in IBD patients.

The next evolution
of the treat-to-target paradigm
Endoscopic healing is known to be an objective measure of therapeutic response.2,3 In multiple clinical trials, endoscopic healing is a therapeutic goal that has been associated with improved long-term outcomes 2-6.
However, current assessment methods can make it challenging to:
- Noninvasively evaluate the endoscopic disease activity 2,7,8
- Assess active disease in difficult-to-reach locations, including the ileum3,6,8
- Obtain objective results for individual patients7
- Regularly monitor patient response to therapy7
Measure and monitor the endoscopic disease activity with one simple serum test
Monitr provides critical information to aid in:
- Establishing a baseline measurement of disease activity at initial presentation
- Tracking and assessing disease activity and response to therapeutic management
- Optimizing treatment management decisions
- Evaluating if treatment changes may be appropriate
- Counseling patients about therapeutic management goals
Monitr is a laboratory-developed test that was developed, and analytically and clinically validated by Prometheus Laboratories Inc. for adult and pediatric inflammatory bowel disease patients under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA-certified and College of American Pathologists-accredited clinical laboratory. As laboratory a developed test, it has not been cleared or approved by the US FDA. This test is not yet available for patients in NY-State. This test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324-1338.
- Alsoud D, Ho J, Sabino J, Ferrante M, Vermeire S, Verstockt B. Utility of the Serum-Based Endoscopic Healing Index in Monitoring Therapeutic Response in Ulcerative Colitis. Am J Gastroenterol. 2023 Oct 19. doi: 10.14309/ajg.0000000000002518.
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779-2789
- De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2013;19(2):429-444.
- D’Haens G, Kelly O, Battat R, et al. Development and validation of a test to monitor endoscopic activity in patients with Crohn’s disease based on serum levels of proteins. Gastroenterology. 2020;158(3):515-526.e10.
- Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142(5):1102-1111.e2.
- Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43(3):317-333.
- Freeman HJ. Limitations in assessment of mucosal healing in inflammatory bowel disease. World J Gastroenterol. 2010;16(1):15-20.
- Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8(12):1582-1597.
