Therapeutic Drug Monitoring

Prometheus® Anser® IFX and PROMETHEUS® Anser® ADA mechanism of detection
As nonradio-labeled liquid-phase mobility shift assays, PROMETHEUS Anser IFX and PROMETHEUS Anser ADA can uniquely measure antibodies to infliximab or antibodies to adalimumab in the presence of infliximab or adalimumab from a single serum sample and at any time.
Antidrug antibody quantification
Drug is tagged with a fluorescent label and incubated with serum. If antibodies are present, the resulting drug antibody complex has a significantly higher molecular weight than free drug, thus allowing the separation of free drug from antibody-bound drug for quantification2,4; the area under the drug antibody peak can be used to quantify the antibody level as shown.
Unlike solid-phase detection methods (for example, ELISA/ECLIA), which can only detect antidrug antibodies that are not bound to circulating drug, the methodology used by PROMETHEUS Anser IFX and PROMETHEUS Anser ADA allows for the detection of both drug-free and drug-bound antidrug antibodies in a patient’s serum. These tests also detect all immunoglobulin subtypes, in addition to antibodies with weak binding affinity – both of which may be missed in ELISA/ECLIA testing.2,4
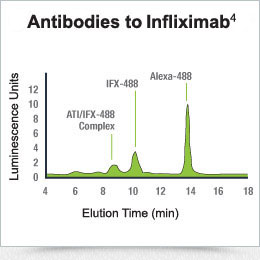
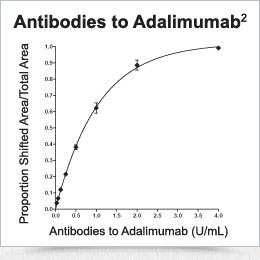
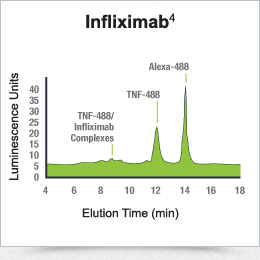
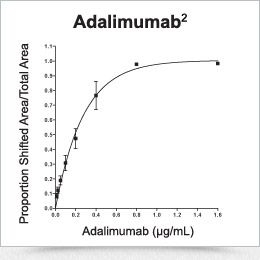
Drug quantification
Fluorescent-labeled TNF-α (the substrate for infliximab and adalimumab) is incubated with serum containing drug. The binding of TNF-α to drug results in a complex that has a much higher molecular weight and can be separated and quantified, as shown.2,4
