Liver

Non-invasively aids in the detection, staging, and monitoring of the severity of liver fibrosis for hepatitis C virus (HCV) patients.
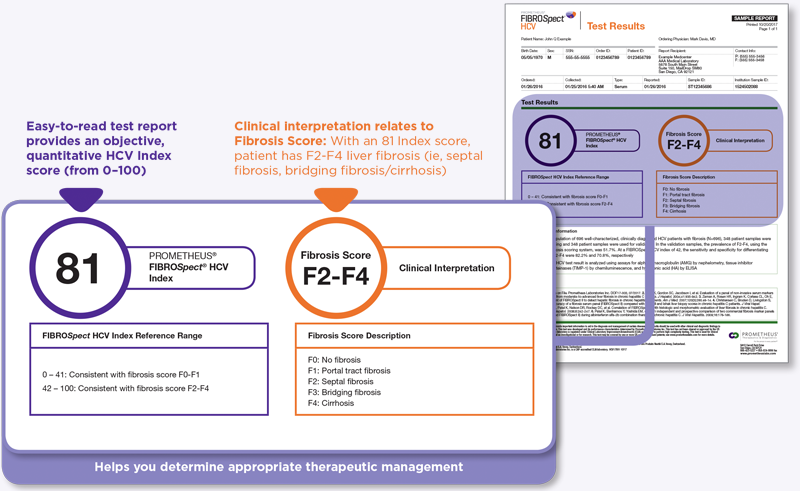
PROMETHEUS® FIBROSpect® HCV
For hepatitis C (HCV) patients, we offer the PROMETHEUS FIBROSpect HCV test, which is a laboratory-developed test that aids in in the detection, staging, and monitoring of liver fibrosis. The simple blood test is noninvasive and provides a quantitative fibrosis score to help physicians risk stratify and monitor patients.
- Differentiates between F0-F1 and F2-F4 liver fibrosis1
- Validated with samples from 348 biopsy-confirmed HCV patients1
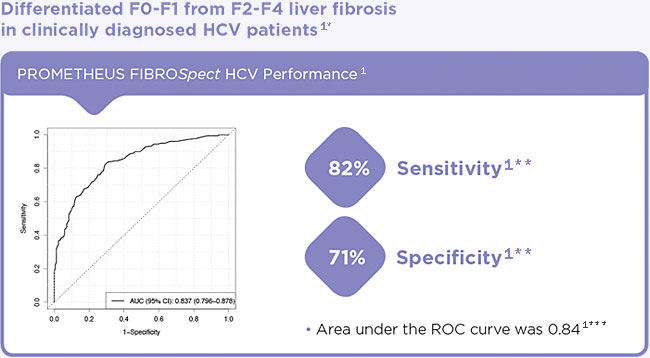
* In a study population of 696 clinically diagnosed HCV patients with liver fibrosis, 348 patient serum samples were used for training and 348 patient serum samples were used for validation.
** At a FIBROSpect HCV Index of 42, the sensitivity and specificity for differentiating F0-F1 from F2-F4 were 82% and 71%, respectively, on the validation samples (n = 348).
***Performance in differentiating F0-F1 from F2-F4 on validation samples (n = 348).
Staging of Liver Fibrosis in HCV Patients is Critical
American Association for Study of Liver Disease (AASLD) and Infectious Disease Society of America (IDSA) 2017 guidelines highlight the benefits of initiating therapy in HCV patients with lower-stage liver fibrosis2:
- “Initiating therapy in patients with lower-stage fibrosis augments the benefits of SVR [sustained virologic response]”
- “Treatment delay may decrease the benefit of SVR”
References
- Data on file. Prometheus Laboratories Inc.
- When and in whom to initiate HCV therapy. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Web site. http://www.hcvguidelines.org/evaluate/when-whom. Last updated July 6, 2016. Accessed July 24, 2017.
FibroSPECT HCV is a laboratory-developed test that were developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA certified (05D0917432) and College of American Pathologists (CAP) accredited (6805501) clinical laboratory. As a laboratory developed test, it has not been cleared or approved by the US FDA. The test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. Prometheus and FibroSPECT are registered trademarks of Prometheus Laboratories Inc, San Diego, California. All other trademarks or service marks are the property of their respective owners. This material is provided for general information purposes only, as an educational service for healthcare providers. It is not intended as a substitute for medical advice and/or consultation with a physician.