Liver

Non-invasively aids in the detection, staging, and monitoring of liver fibrosis for nonalcoholic steatohepatitus (NASH) patients.
Introduction to NASH
Nonalcoholic steatohepatitis (NASH) affects approximately 25 million people in the US.1 In 2019 NASH was the second leading indication, and the indication increasing with the fastest rate, for liver transplantation.2 Currently, biopsy is the gold standard for the diagnosis of NASH. However, biopsy may be subject to sampling problems that can confound the results, in addition to surgical costs, risk, and inconvenience for the patient.
Identifying High-Risk NASH Patients is Imperative
- F3-F4 stage of liver fibrosis has been identified as the most significant independent risk factor for mortality in NASH patients3,4
- NASH-related cirrhosis is becoming a more common indication for liver transplantation3
- Progression of NASH-related liver fibrosis is 2 times faster than nonalcoholic fatty liver disease5
PROMETHEUS® FIBROSpect® NASH
PROMETHEUS FIBROSpect NASH is a laboratory-developed test that aids in in the detection, staging, and monitoring of liver fibrosis in nonalcoholic steatohepatitis patients. The simple blood test is noninvasive and provides a quantitative fibrosis score to help physicians risk stratify and monitor patients based on 3 clinically relevant biomarkers.

Proprietary NASH-specific algorithm
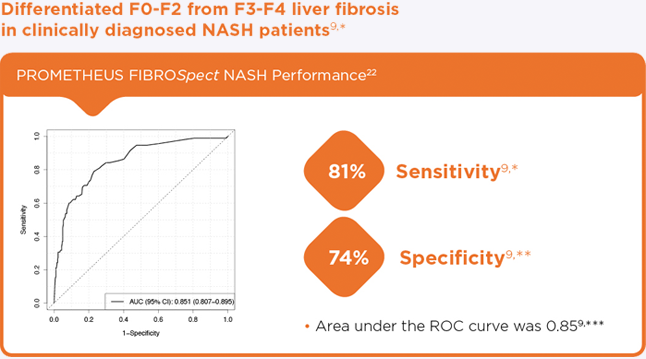
- Differentiated F0-F2 from F3-F4 liver fibrosis in clinically diagnosed NASH patients9
- Validated with samples from 396 biopsy-confirmed NASH patients9
- Provides quantitative liver fibrosis test results
FIBROSpect NASH benefits:
- Easy-to-read and interpret test result
- Noninvasive and non-fasting
- Can be repeated frequently, easily, and safely
- May reduce the need for biopsy
References
- National Institute of Diabetes and Digestive and Kidney Diseases Web site. Definition & facts of NAFLD & NASH. https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts. Accessed August 7, 2017
- Younossi Z, Stepanova M, Ong J, et al. Nonalcoholic Steatophepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gasteroenterol Hepatol. 2021;19(3)580-589.
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
- Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-1554.
- Jayakumar S, Harrison SA, Loomba R. Noninvasive markers of fibrosis and inflammation in nonalcoholic fatty liver disease. Curr Hepatol Rep. 2016;15(2):86-95.
- Boeker KH, Haberkorn CI, Michels D, Flemming P, Manns MP, Lichtinghagen R. Diagnostic potential of circulating TIMP-1 and MMP-2 as markers of liver fibrosis in patients with chronic hepatitis C. Clin Chim Acta. 2002;316(1-2):71-81.
- Halfon P, Bourlière M, Pénaranda G, et al. Accuracy of hyaluronic acid level for predicting liver fibrosis stages in patients with hepatitis C virus. Comp Hepatol. 2005;4:6.
- Ho AS, Cheng CC, Lee SC, et al. Novel biomarkers predict liver fibrosis in hepatitis C patients: alpha 2 macroglobulin, vitamin D binding protein and apolipoprotein AI. J Biomed Sci. 2010;17(58):1-7.
- Data on file. Prometheus Laboratories Inc.
- When and in whom to initiate HCV therapy. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Web site. http://www.hcvguidelines.org/evaluate/when-whom. Last updated July 6, 2016. Accessed July 24, 2017.
FibroSPECT HCV is a laboratory-developed test that were developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA certified (05D0917432) and College of American Pathologists (CAP) accredited (6805501) clinical laboratory. As a laboratory developed test, it has not been cleared or approved by the US FDA. The test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. Prometheus and FibroSPECT are registered trademarks of Prometheus Laboratories Inc, San Diego, California. All other trademarks or service marks are the property of their respective owners. This material is provided for general information purposes only, as an educational service for healthcare providers. It is not intended as a substitute for medical advice and/or consultation with a physician.

