Precision-Guided Dosing


Biologic dosing is not one size fits all
The unpredictable pharmacokinetic variability of biologic therapies amongst individual
inflammatory bowel disease (IBD) patients makes it extremely difficult to achieve
therapeutic targets quickly and precisely.1
• Standard dosing is inadequate for many patients2,3
• Current tests provide a single point-in-time snapshot on drug levels, but don’t provide insights on dosage or interval to achieve targets.
Conventional dosing poses many challenges for providers and patients alike.4-6
• Suboptimal therapeutic levels
• Potential loss of response
• Inefficient care
• Negative impact on quality of life
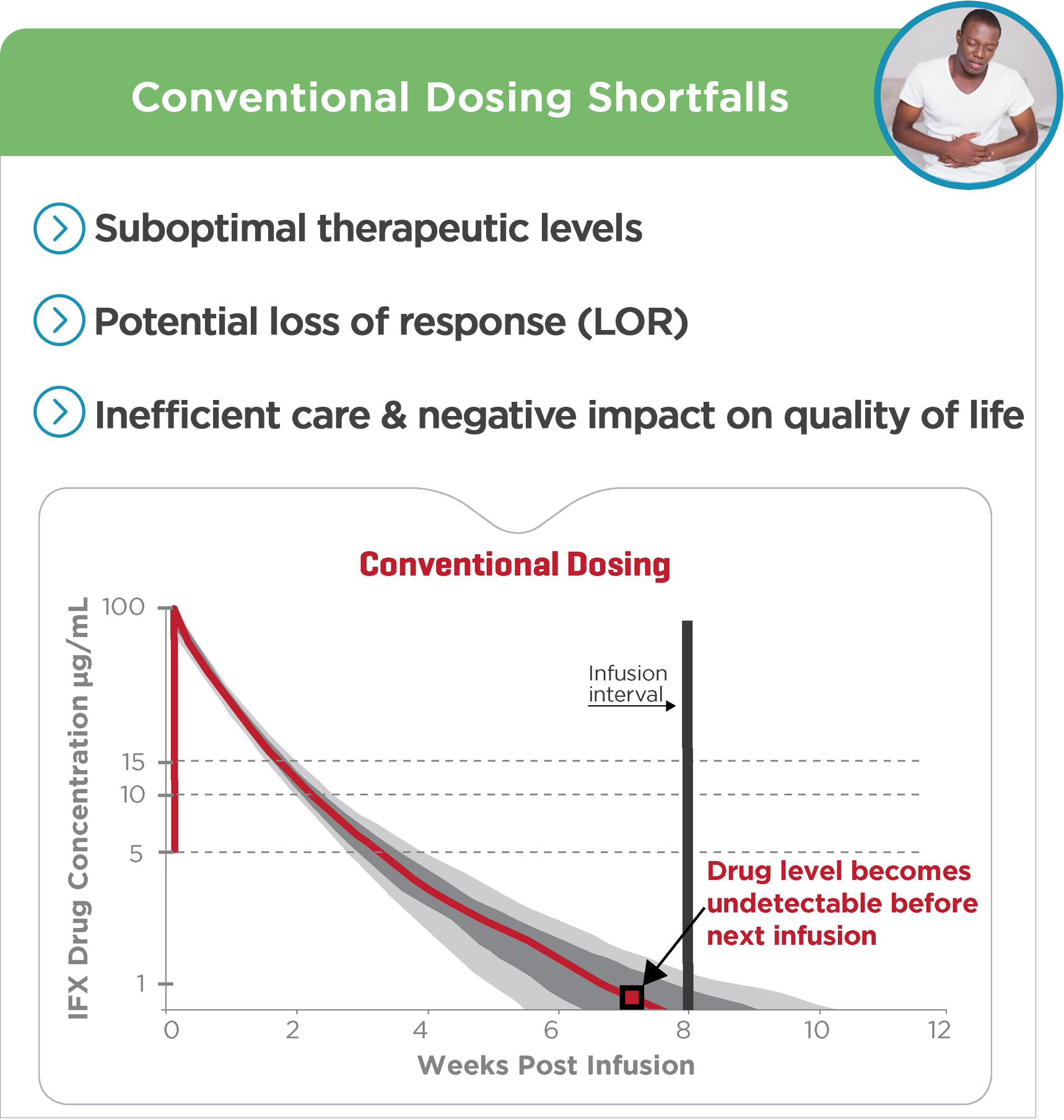
Conventional approaches to drug monitoring can lead to unoptimized therapy
resulting in inefficient care, disease recurrence and potential treatment failure
Control the trajectory of therapeutic drug levels with precision-guided care
PredictrPK®, available exclusively from Prometheus Laboratories, offers individualized, actionable evidence to expediently fine-tune ustekinumab (UST) dosing in support of durable response and sustained clinical remission. These paradigm shifting tests help guide biologic optimization and aid in controlling the trajectory of therapeutic drug levels and achieving treatment targets.


PredictrPK provides objective, individualized predictions of optimal biologic dosing for UST that empowers providers to precisely:
• Accelerate treatment optimization supporting drug persistence
• Increase certainty of achieving therapeutic targets
• Improved patient confidence and satisfaction
Evidence-based outcomes of dosing optimization:4-9
• Durability of response
• Improved remission rates
• Reduced healthcare consumption
• Improved quality of life and efficient care
Pharmacokinetically-guided insights at every stage of therapy
provides evidence to validate treatment plans and empower individualized care
PredictrPK UST maintenance is a laboratory-developed test that was developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines for adult IBD patients undergoing maintenance ustekinumab therapy, and is performed exclusively in our high complexity CLIA-certified and College of American Pathologists-accredited clinical laboratory. As laboratory a developed test, it has not been cleared or approved by the US FDA. This test is not yet available for patients in NY-State. This test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Vermeire et al. Clin Gastroenterol Hepatol. 2020; 18:1291–1299.
- Rutgeerts P et al. Gastroenterology. 2018;155:1045–1058.
- Sandborn WJ et al. N Engl J Med. 2012;367:1519–1528.
- Negoescu et al. Inflamm Bowel Dis. 2020; 26:103-111.
- Papamichael et al. J Crohns Colitis. 2018; 12:804-810.
- Papamichael et al. Clin Gastroenterol Hepatol. 2017; 15:1580-1588.
- Strik et al. Scand J Gastroenterol. 2021; 56:145-154.
- Dubinsky et al. Inflamm Bowel Dis. 2022; 28:1375-1385.
- Primas et al. J Clin Med. 2022; 11:3316.
- C Law et al. J Crohn’s and Colitis. 2024; 18(S1): i1102–i110.
