Precision-Guided Therapy Selection

![[Page Header]_Respondr_TNF_main_page_2025 Female with IBD. Is the prescribed biologic right for her?](https://prometheuslabs.com/wp-content/uploads/2025/04/Page-Header_Respondr_TNF_main_page_2025.png)
Unpredictable response can make biologic therapies challenging
Anti-TNF biologics have been shown to be highly effective for IBD patients, but the variability in response can make the process for patients to achieve and maintain remission burdensome.
Common factors impacting drug exposure and efficacy:
- Accelerated drug clearance1-2
- Antidrug antibody development driven by pharmacokinetics and/or genetic predisposition1-4

Early selection of the right therapeutic and effective management improves patient outcomes
By optimizing the selection and management of the first therapeutic, patients experience:
- Better disease control and an increased likelihood of achieving sustained remission5-7
- Reduced risk of surgery or hospitalizations, and improved quality of life5-7
Tools to confidently select an appropriate therapy plan before initiation have been limited — until now.

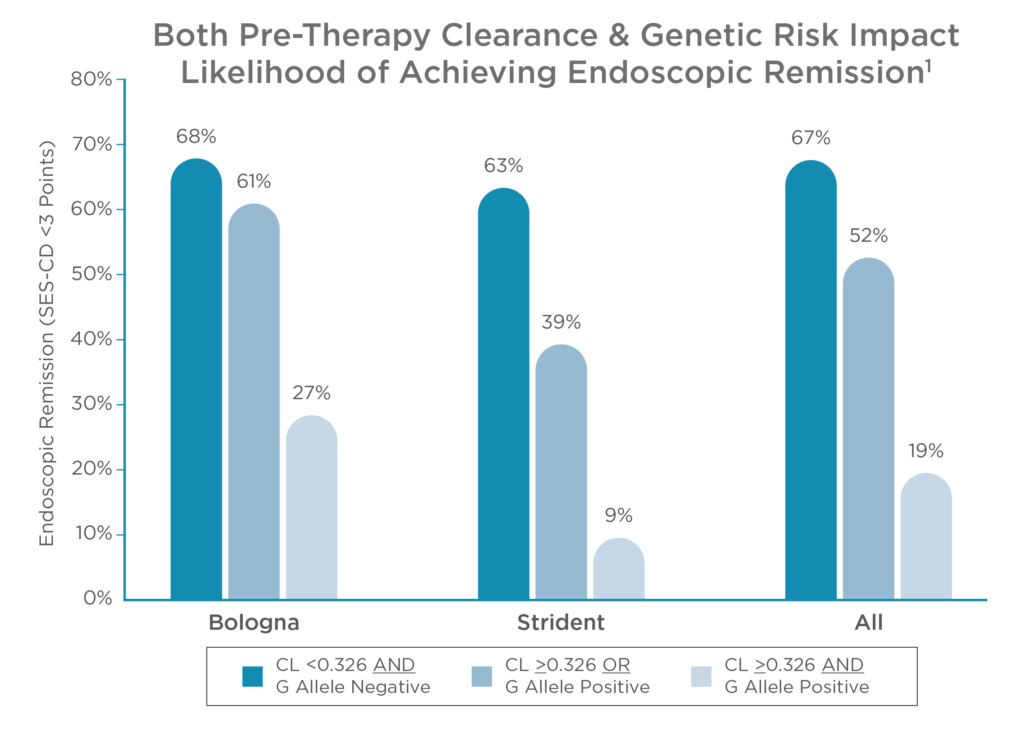
Pre-therapy clearance and genetic risk can predict a patient’s response to anti-TNFs
Accelerated pre-therapy clearance (>0.326 L/day) and carriage of the HLA-DQA1*05 G allele significantly impact therapy response.1 Patients with these factors are:
- 2x more likely to develop antidrug antibodies1
- Less likely to achieve clinical, biochemical or endoscopic remission1,4,8
Identifying patient risk factors prior to therapy selection enables treatment plans to be personalized to the IBD patient.
Respondr TNF is a laboratory-developed test that was developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA-certified (05D0917432) and College of American Pathologists-accredited (6805501) clinical laboratory. As laboratory a developed test, it has not been cleared or approved by the US FDA. This test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Spencer et al. Front Immunol. 2024 Jan 23;15:1342477.
- Dubinsky M et al. Pharmaceutics. 2023 Sep 30;15(10):2408.
- Sazonovs et al. Gastroenterology. 2020 Jan;158(1):189-199.
- Wilson A et al. Aliment Pharmacol Ther. 2020 Feb;51(3):356-363.
- Danese S et al. Gut. 2017; 66, 2179–2187.
- Solitano V et al. J Clin Med. 2020 Aug 14;9(8):2646.
- Colombel JC et al. Gastroenterology. 2017 Feb;152(2):351-361.e5.
- McGovern et al. Gastroenterology. 2023 May;164(6):S-1117-1118.

