Inflammatory Bowel Disease

Goes beyond just inflammatory processes
In developing the Monitr® Crohn’s Disease Test, Prometheus evaluated numerous factors that are related to mucosal damage and healing processes, including7:
- Inflammatory bowel disease (IBD) biology
- Relevant IBD signaling pathways
- Therapeutic targets
- Tumor biology
- Classic wound healing
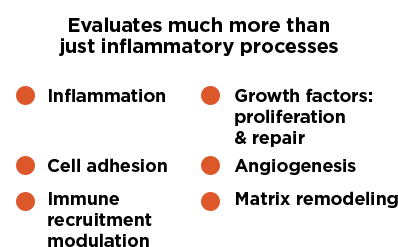
Monitr provides healthcare professionals with more information than inflammatory biomarkers alone. This innovative, laboratory-developed test features 13 biomarkers that are associated with mucosal damage and repair processes, including7:
- Inflammation: high-sensitivity C-reactive protein (hsCRP) and serum amyloid A 1 (SAA 1)
- Cell adhesion: carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM 1) and vascular cell adhesion molecule 1 (VCAM 1)
- Immune recruitment modulation: interleukin-7 (IL-7)
- Growth factors (proliferation & repair): transforming growth factor α (TGFα)
- Angiogenesis: angiopoietin 1 (Ang 1) and Ang 2
- Matrix remodeling: matrix metalloproteinase 1 (MMP 1), MMP 2, MMP 3, MMP 9, and extracellular matrix metalloproteinase inducer (EMMPRIN)
Biomarkers are clinically valuable, but currently have limitations1-4
C-reactive protein (CRP) is:
- NOT associated with high sensitivity3,*
- NOT gut specific for inflammation5
- NOT elevated in as many as 25% of patients with active Crohn’s disease (CD): (“CRP nonresponders”)4
Fecal calprotectin (FC) is:
- NOT CD specific6
- NOT preferred (poor patient compliance)7,8
Easy to order: Monitr Test Requisition Form. For assistance ordering,
contact Client Services toll-free in the US at 888-423-5227 (Monday through Friday from 6 AM to 4:30 PM PT).
*In a review and meta-analysis of 19 studies, pooled sensitivity and specificity estimates for CRP were 0.49 and 0.92, respectively.
References
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324-1338.
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779-2789.
- Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(6):802-819.
- Jürgens M, Mahachie John JM, Cleynen I, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9(5):421-427.e1.
- Walsham NE, Sherwood RA. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol. 2016;9:21-29.
- Bjarnason I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2017;13(1):53-56.
- D’Haens G, Kelly O, Battat R, et al. Development and validation of a test to monitor endoscopic activity in patients with Crohn’s disease based on serum levels of proteins. Gastroenterology. 2020;158(3):515-526.e10.
- Shah JM, Omar E, Pai DR, Sood S. Cellular events and biomarkers of wound healing. Indian J Plast Surg. 2012;45(2):220-228.
