Precision-Guided Therapy Selection

Respondr® TNF reports provide objective guidance for therapy selection and strategic patient management
Test results help stratify each patient by their ability to achieve and sustain a response to anti-TNF therapy and identify patients who may benefit from more active disease management to achieve therapy goals.
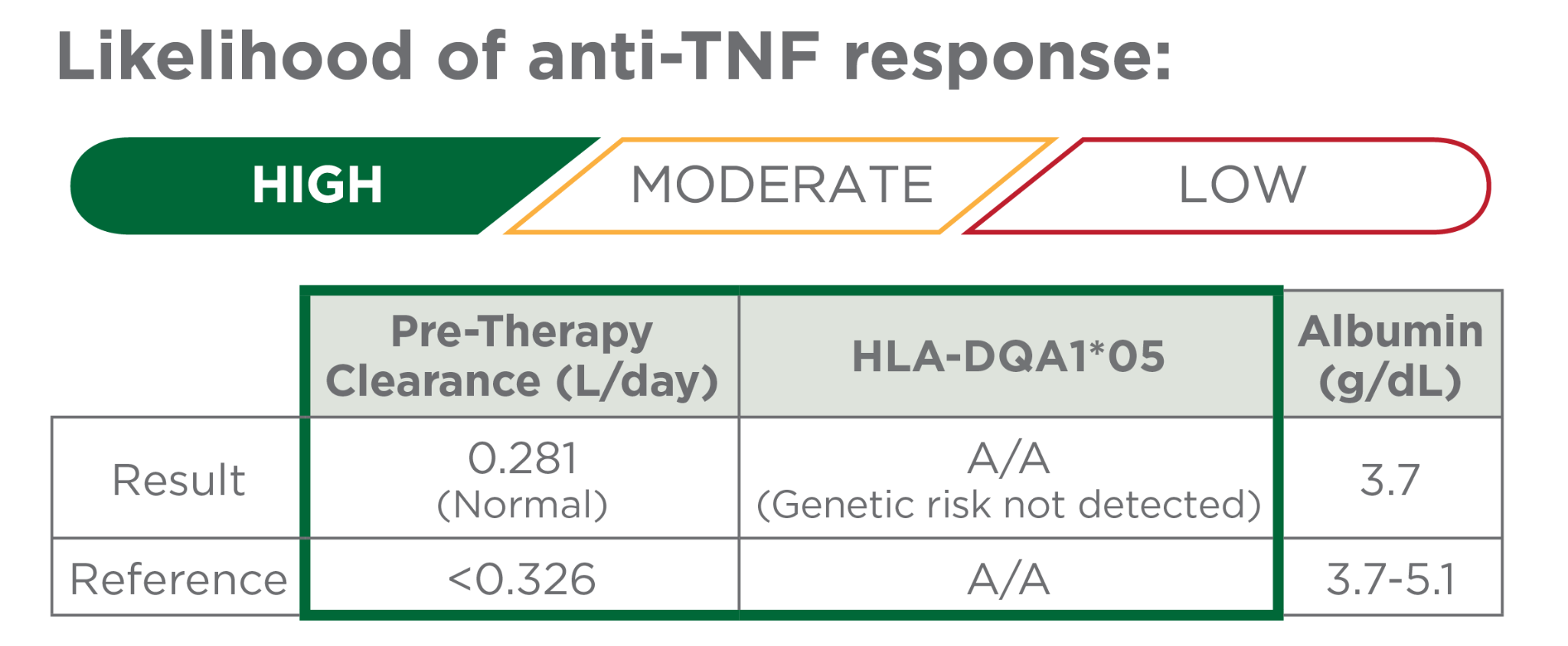
Risk factors not detected
Early precision-guided dosing associated with a greater likelihood of remission.1-4,10
- There is a 91-95% probability of week 14 clinical remission with precision-guided dosing vs. 46-60% with standard dosing
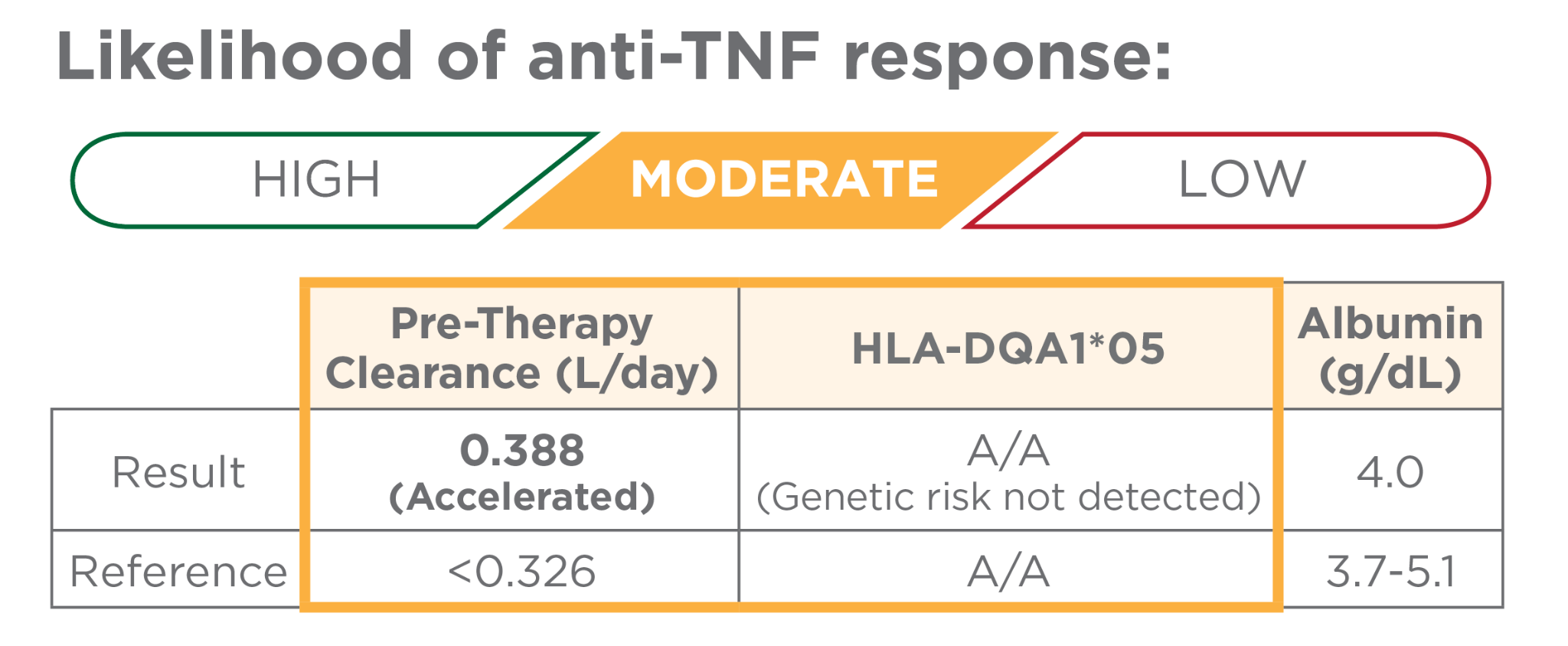
Accelerated pre-therapy clearance OR genetic risk detected
Precision-guided dosing should be considered to reduce the risks of immunogenicity and biologic failure, and an increase the likelihood of response to infliximab or adalimumab.1-6,10
- Accelerated pre-therapy clearance and genetic risk each impart a 2-fold increased likelihood of immunogenicity and lack of disease control
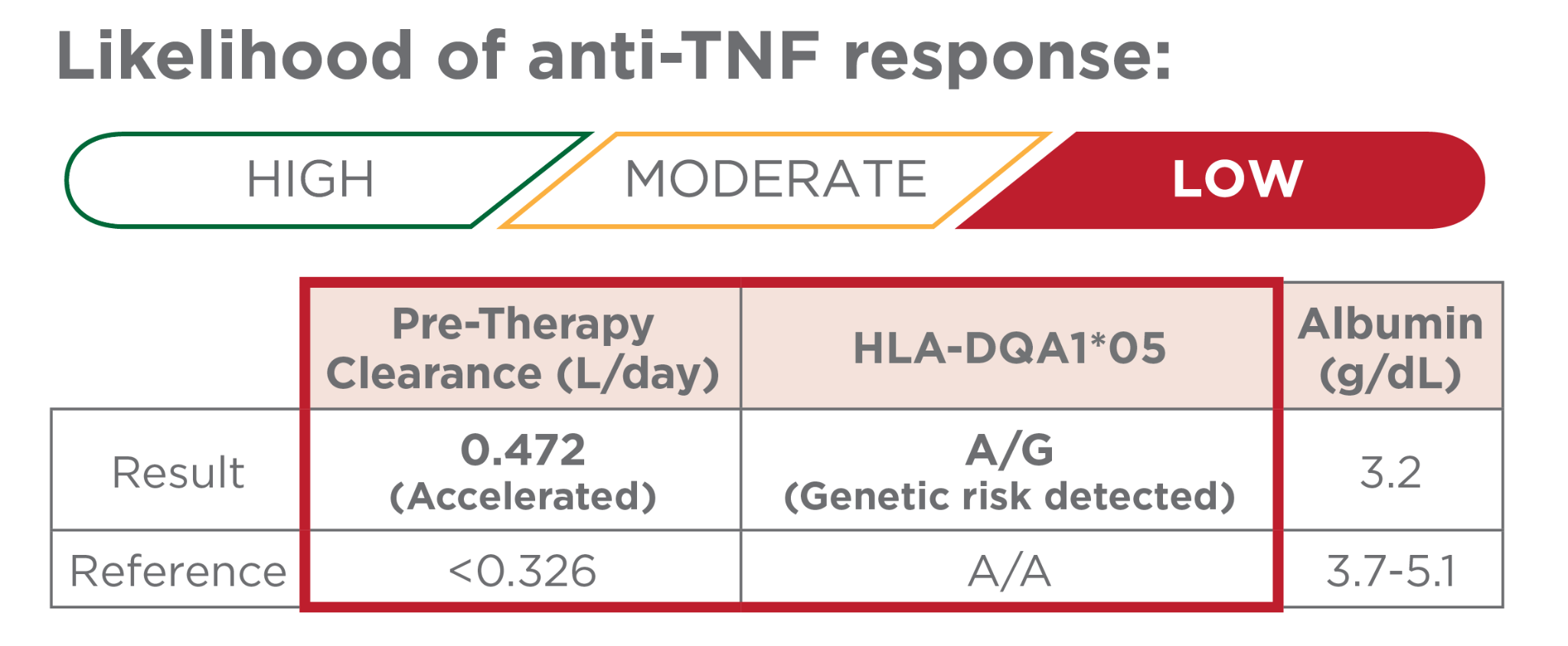
Both accelerated pre-therapy clearance AND genetic risk detected
Early precision-guided dosing should be highly considered to increase the likelihood of achieving and sustaining a therapeutic response.1,5-8
- Hospitalized acute severe ulcerative colitis patients with significantly accelerated clearance have an increased risk of colectomy7
What’s Next?
Providers may want to consider PredictrPK IFX induction or PredictrPK ADA maintenance for dosing optimization or Thiopurine Metabolites for monitoring treatment.
Respondr TNF is a laboratory-developed test that was developed, and analytically and clinically validated by Prometheus Laboratories Inc. under federal Clinical Laboratory Improvement Amendments (CLIA) guidelines, and is performed exclusively in our high complexity CLIA-certified (05D0917432) and College of American Pathologists-accredited (6805501) clinical laboratory. As laboratory a developed test, it has not been cleared or approved by the US FDA. This test may be covered by one or more US pending or issued patents – see prometheuslabs.com/patents. This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
References
- Spencer et al. Front Immunol. 2024;15:doi:10.3389/fimmu.2024.1342477.
- Spencer E et al. Gastroenterology. 2022 May;162(6):1746-1748.e3.
- Dubinsky M et al. Pharmaceutics. 2023 Sep 30;15(10):2408.
- Hanauer S et al. Pharmaceutics. 2025;17(4): 428.
- Sazonovs et al. Gastroenterology. 2020;158:189-199.
- Wilson A et al. Aliment Pharmacol Ther. 2020 Feb;51(3):356-363.
- Battat R et al. Clin Gastroenterol Hepatol. 2021 Mar;19(3):511-518.e6.
- McGovern et al. Gastroenterology. 2023 May;164(6):S-1117-1118.
- Danese S et al. Gut. 2017; 66, 2179–2187.
- Data on File
